Saccharomyces cerevisiae as a Model to Study Replicative Senescence Triggered by Telomere Shortening
- PMID: 23638436
- PMCID: PMC3636481
- DOI: 10.3389/fonc.2013.00101
Saccharomyces cerevisiae as a Model to Study Replicative Senescence Triggered by Telomere Shortening
Abstract
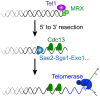
In many somatic human tissues, telomeres shorten progressively because of the DNA-end replication problem. Consequently, cells cease to proliferate and are maintained in a metabolically viable state called replicative senescence. These cells are characterized by an activation of DNA damage checkpoints stemming from eroded telomeres, which are bypassed in many cancer cells. Hence, replicative senescence has been considered one of the most potent tumor suppressor pathways. However, the mechanism through which short telomeres trigger this cellular response is far from being understood. When telomerase is removed experimentally in Saccharomyces cerevisiae, telomere shortening also results in a gradual arrest of population growth, suggesting that replicative senescence also occurs in this unicellular eukaryote. In this review, we present the key steps that have contributed to the understanding of the mechanisms underlying the establishment of replicative senescence in budding yeast. As in mammals, signals stemming from short telomeres activate the DNA damage checkpoints, suggesting that the early cellular response to the shortest telomere(s) is conserved in evolution. Yet closer analysis reveals a complex picture in which the apparent single checkpoint response may result from a variety of telomeric alterations expressed in the absence of telomerase. Accordingly, the DNA replication of eroding telomeres appears as a critical challenge for senescing budding yeast cells and the easy manipulation of S. cerevisiae is providing insights into the way short telomeres are integrated into their chromatin and nuclear environments. Finally, the loss of telomerase in budding yeast triggers a more general metabolic alteration that remains largely unexplored. Thus, telomerase-deficient S. cerevisiae cells may have more common points than anticipated with somatic cells, in which telomerase depletion is naturally programed, thus potentially inspiring investigations in mammalian cells.
Keywords: DNA damage checkpoints; DNA replication; Saccharomyces cerevisiae; replicative senescence; telomerase; telomeres.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

