Functional interaction between pre-synaptic α6β2-containing nicotinic and adenosine A2A receptors in the control of dopamine release in the rat striatum
- PMID: 23638679
- PMCID: PMC3724115
- DOI: 10.1111/bph.12234
Functional interaction between pre-synaptic α6β2-containing nicotinic and adenosine A2A receptors in the control of dopamine release in the rat striatum
Abstract
Background and purpose: Pre-synaptic nicotinic ACh receptors (nAChRs) and adenosine A2A receptors (A2A Rs) are involved in the control of dopamine release and are putative therapeutic targets in Parkinson's disease and addiction. Since A2A Rs have been reported to interact with nAChRs, here we aimed at mapping the possible functional interaction between A2A Rs and nAChRs in rat striatal dopaminergic terminals.
Experimental approach: We pharmacologically characterized the release of dopamine and defined the localization of nAChR subunits in rat striatal nerve terminals in vitro and carried out locomotor behavioural sensitization in rats in vivo.
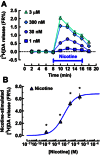
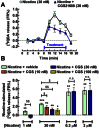
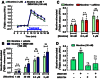
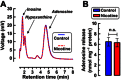
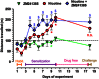
Key results: In striatal nerve terminals, the selective A2A R agonist CGS21680 inhibited, while the A2A R antagonist ZM241385 potentiated the nicotine-stimulated [(3) H]dopamine ([(3) H]DA) release. Upon blockade of the α6 subunit-containing nAChRs, the remaining nicotine-stimulated [(3) H]DA release was no longer modulated by A2A R ligands. In the locomotor sensitization experiments, nicotine enhanced the locomotor activity on day 7 of repeated nicotine injection, an effect that no longer persisted after 1 week of drug withdrawal. Notably, ZM241385-injected rats developed locomotor sensitization to nicotine already on day 2, which remained persistent upon nicotine withdrawal.
Conclusions and implications: These results provide the first evidence for a functional interaction between nicotinic and adenosine A2A R in striatal dopaminergic terminals, with likely therapeutic consequences for smoking, Parkinson's disease and other dopaminergic disorders.
Keywords: adenosine; adenosine A2A receptor; adenosine A2B receptor; dopamine; locomotor sensitization; nAChRs; nicotine; rat; striatum.
© 2013 The British Pharmacological Society.
Figures


References
-
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Köfalvi A, Panlilio L, et al. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. - PubMed
-
- Calabresi P, Di Filippo M. ACh/dopamine crosstalk in motor control and reward: a crucial role for alpha 6-containing nicotinic receptors? Neuron. 2008;60:4–7. - PubMed
-
- Castañé A, Soria G, Ledent C, Maldonado R, Valverde O. Attenuation of nicotine-induced rewarding effects in A2A knockout mice. Neuropharmacology. 2006;51:631–640. - PubMed
-
- Celik E, Uzbay IT, Karakas S. Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:50–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

