Elective lymph node irradiation late course accelerated hyper-fractionated radiotherapy plus concurrent cisplatin-based chemotherapy for esophageal squamous cell carcinoma: a phase II study
- PMID: 23638721
- PMCID: PMC3653710
- DOI: 10.1186/1748-717X-8-108
Elective lymph node irradiation late course accelerated hyper-fractionated radiotherapy plus concurrent cisplatin-based chemotherapy for esophageal squamous cell carcinoma: a phase II study
Abstract
Background: In this phase II study, we evaluated the efficacy, toxicity, and patterns of failure of elective lymph node irradiation (ENI) late course accelerated hyper-fractionated radiotherapy (LCAHRT) concurrently with cisplatin-based chemotherapy (CHT) for esophageal squamous cell carcinoma (ESCC).
Methods: Patients with clinical stage II-IVa (T1-4N0-1M0 or M1a) ESCC were enrolled between 2004 and 2011. Radiation therapy (RT) comprised two courses: The first course of radiation covered the primary and metastatic regional tumors and high risk lymph nodal regions, given at 2 Gy per fraction for a dose of 40 Gy. In the second course, LCAHRT was delivered to the boost volume twice a day for an additional 19.6 Gy in 7 treatment days, using 1.4 Gy per fraction. Two cycles of CHT were given at the beginning of RT.
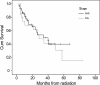
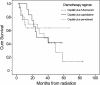
Results: The median age and Karnofsky performance status were 63 years and 80, respectively. The American Joint Committee on Cancer stage was II in 14 (20.6%) patients, III in 32 (47.1%), and IVa in 22 (32.3%). With a median follow-up of 18.5 months, the overall survival at 1-, 3-, 5-year were 75.5%, 46.5%, 22.7% for whole group patients, versus 78.6%, 49.4%, 39.9% for patients with stage II-III. The patterns of first failure from local recurrence, regional failure, and distant metastasis were seen in 20.6%, 17.6%, and 19.1%, respectively. The most frequent acute high-grade (≥ 3) toxicities were esophagitis and leucopenia, occurred in 26.4% and 32.4%.
Conclusions: ENI LCAHRT concurrently with CHT was appeared to be an effective regimen for ESCC patient with a favorable and tolerated profile. Further observation with longer time and randomized phase III trial is currently underway.
Trial registration: ChiCTR-TRC-09000568.
Figures


Similar articles
-
Concurrent Selective Lymph Node Radiotherapy and S-1 Plus Cisplatin for Esophageal Squamous Cell Carcinoma: A Phase II Study.Ann Surg Oncol. 2019 Jun;26(6):1886-1892. doi: 10.1245/s10434-019-07264-4. Epub 2019 Feb 25. Ann Surg Oncol. 2019. PMID: 30805810 Clinical Trial.
-
Phase II study of concurrent selective lymph node late course accelerated hyper-fractionated radiotherapy and pemetrexed and cisplatin for locally advanced oesophageal squamous cell carcinoma.Br J Radiol. 2014 May;87(1037):20130656. doi: 10.1259/bjr.20130656. Epub 2014 Mar 26. Br J Radiol. 2014. PMID: 24666012 Free PMC article. Clinical Trial.
-
Phase II feasibility study of preoperative concurrent chemoradiotherapy with cisplatin plus 5-fluorouracil and elective lymph node irradiation for clinical stage II/III esophageal squamous cell carcinoma.Int J Clin Oncol. 2019 Jan;24(1):60-67. doi: 10.1007/s10147-018-1336-x. Epub 2018 Aug 14. Int J Clin Oncol. 2019. PMID: 30109544 Clinical Trial.
-
Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice.Radiat Oncol. 2016 Feb 5;11:18. doi: 10.1186/s13014-016-0589-7. Radiat Oncol. 2016. PMID: 26846932 Free PMC article. Review.
-
Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix.Int J Radiat Oncol Biol Phys. 2003 Apr 1;55(5):1247-53. doi: 10.1016/s0360-3016(02)04401-2. Int J Radiat Oncol Biol Phys. 2003. PMID: 12654434 Review.
Cited by
-
Analysis of different fractionations of three-dimensional conformable radiotherapy for esophageal cancer.Int J Clin Exp Med. 2015 Jul 15;8(7):11139-45. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26379915 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

