Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data
- PMID: 23639488
- PMCID: PMC3671272
- DOI: 10.1016/S0140-6736(13)60140-3
Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data
Abstract
Background: Tamoxifen and raloxifene reduce the risk of breast cancer in women at elevated risk of disease, but the duration of the effect is unknown. We assessed the effectiveness of selective oestrogen receptor modulators (SERMs) on breast cancer incidence.
Methods: We did a meta-analysis with individual participant data from nine prevention trials comparing four selective oestrogen receptor modulators (SERMs; tamoxifen, raloxifene, arzoxifene, and lasofoxifene) with placebo, or in one study with tamoxifen. Our primary endpoint was incidence of all breast cancer (including ductal carcinoma in situ) during a 10 year follow-up period. Analysis was by intention to treat.
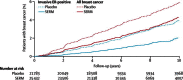
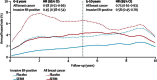
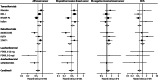
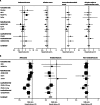
Results: We analysed data for 83,399 women with 306,617 women-years of follow-up. Median follow-up was 65 months (IQR 54-93). Overall, we noted a 38% reduction (hazard ratio [HR] 0·62, 95% CI 0·56-0·69) in breast cancer incidence, and 42 women would need to be treated to prevent one breast cancer event in the first 10 years of follow-up. The reduction was larger in the first 5 years of follow-up than in years 5-10 (42%, HR 0·58, 0·51-0·66; p<0·0001 vs 25%, 0·75, 0·61-0·93; p=0·007), but we noted no heterogeneity between time periods. Thromboembolic events were significantly increased with all SERMs (odds ratio 1·73, 95% CI 1·47-2·05; p<0·0001). We recorded a significant reduction of 34% in vertebral fractures (0·66, 0·59-0·73), but only a small effect for non-vertebral fractures (0·93, 0·87-0·99).
Interpretation: For all SERMs, incidence of invasive oestrogen (ER)-positive breast cancer was reduced both during treatment and for at least 5 years after completion. Similar to other preventive interventions, careful consideration of risks and benefits is needed to identify women who are most likely to benefit from these drugs.
Funding: Cancer Research UK.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Breast cancer prevention: SERMs come of age.Lancet. 2013 May 25;381(9880):1795-7. doi: 10.1016/S0140-6736(13)60443-2. Epub 2013 Apr 30. Lancet. 2013. PMID: 23639489 No abstract available.
-
Meta-analysis: selective estrogen-receptor modulators reduce breast cancer incidence.Ann Intern Med. 2013 Sep 17;159(6):JC9. doi: 10.7326/0003-4819-159-6-201309170-02009. Ann Intern Med. 2013. PMID: 24042392 No abstract available.
References
-
- Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2:282. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. - PubMed
-
- Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46–53. - PubMed
-
- Cuzick J, Powles T, Veronesi U. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. - PubMed
-
- Powles T, Eeles R, Ashley S. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352:98–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

