What the clock tells the eye: lessons from an ancient arthropod
- PMID: 23639718
- PMCID: PMC4031653
- DOI: 10.1093/icb/ict020
What the clock tells the eye: lessons from an ancient arthropod
Abstract
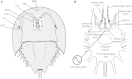
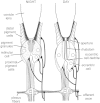
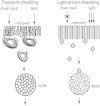
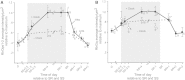
Circadian changes in visual sensitivity have been observed in a wide range of species, vertebrates, and invertebrates, but the processes impacted and the underlying mechanisms largely are unexplored. Among arthropods, effects of circadian signals on vision have been examined in most detail in the lateral compound eye (LE) of the American horseshoe crab, Limulus polyphemus, a chelicerate arthropod. As a consequence of processes influenced by a central circadian clock, Limulus can see at night nearly as well as they do during the day. The effects of the clock on horseshoe crab LE retinas are diverse and include changes in structure, gene expression, and rhabdom biochemistry. An examination of the known effects of circadian rhythms on LEs shows that the effects have three important outcomes: an increase in visual sensitivity at night, a rapid decrease in visual sensitivity at dawn, and maintenance of eyes in a relatively low state of sensitivity during the day, even in the dark. All three outcomes may be critically important for species' survival. Specific effects of circadian rhythms on vision will certainly vary with species and according to life styles. Studies of the circadian regulation of Limulus vision have revealed that these effects can be extremely diverse and profound and suggest that circadian clocks can play a critical role in the ability of animals to adapt to the dramatic daily changes in ambient illumination.
Figures


Similar articles
-
Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: II. Relationship to circadian rhythms of visual sensitivity.Biol Bull. 2008 Aug;215(1):46-56. doi: 10.2307/25470682. Biol Bull. 2008. PMID: 18723636
-
Central regulation of photosensitive membrane turnover in the lateral eye of Limulus. I. Octopamine primes the retina for daily transient rhabdom shedding.Vis Neurosci. 2002 May-Jun;19(3):283-97. doi: 10.1017/s0952523802192066. Vis Neurosci. 2002. PMID: 12392178
-
Central regulation of photosensitive membrane turnover in the lateral eye of Limulus, II: octopamine acts via adenylate cyclase/cAMP-dependent protein kinase to prime the retina for transient rhabdom shedding.Vis Neurosci. 2004 Sep-Oct;21(5):749-63. doi: 10.1017/s0952523804215097. Vis Neurosci. 2004. PMID: 15688551
-
Opsins and Their Expression Patterns in the Xiphosuran Limulus polyphemus.Biol Bull. 2017 Aug;233(1):3-20. doi: 10.1086/693730. Epub 2017 Oct 31. Biol Bull. 2017. PMID: 29182506 Review.
-
Simple Eyes, Extraocular Photoreceptors and Opsins in the American Horseshoe Crab.Integr Comp Biol. 2016 Nov;56(5):809-819. doi: 10.1093/icb/icw093. Epub 2016 Jul 21. Integr Comp Biol. 2016. PMID: 27444526 Review.
Cited by
-
Opsins in Limulus eyes: characterization of three visible light-sensitive opsins unique to and co-expressed in median eye photoreceptors and a peropsin/RGR that is expressed in all eyes.J Exp Biol. 2015 Feb 1;218(Pt 3):466-79. doi: 10.1242/jeb.116087. Epub 2014 Dec 18. J Exp Biol. 2015. PMID: 25524988 Free PMC article.
-
Environmental cues and symbiont microbe-associated molecular patterns function in concert to drive the daily remodelling of the crypt-cell brush border of the Euprymna scolopes light organ.Cell Microbiol. 2016 Nov;18(11):1642-1652. doi: 10.1111/cmi.12602. Epub 2016 May 3. Cell Microbiol. 2016. PMID: 27062511 Free PMC article.
-
Cerebral photoreception in mantis shrimp.Sci Rep. 2018 Jun 26;8(1):9689. doi: 10.1038/s41598-018-28004-w. Sci Rep. 2018. PMID: 29946145 Free PMC article.
-
The expression of three opsin genes from the compound eye of Helicoverpa armigera (Lepidoptera: Noctuidae) is regulated by a circadian clock, light conditions and nutritional status.PLoS One. 2014 Oct 29;9(10):e111683. doi: 10.1371/journal.pone.0111683. eCollection 2014. PLoS One. 2014. PMID: 25353953 Free PMC article.
-
Identification of putative circadian clock genes in the American horseshoe crab, Limulus polyphemus.Comp Biochem Physiol Part D Genomics Proteomics. 2016 Sep;19:45-61. doi: 10.1016/j.cbd.2016.06.001. Epub 2016 Jun 8. Comp Biochem Physiol Part D Genomics Proteomics. 2016. PMID: 27341138 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

