Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells
- PMID: 23639729
- PMCID: PMC3679295
- DOI: 10.1016/j.ydbio.2013.04.021
Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells
Abstract
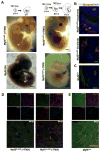
Myf5 is a member of the muscle-specific determination genes and plays a critical role in skeletal muscle development. Whereas the expression of Myf5 during embryonic and fetal myogenesis has been extensively studied, its expression in progenitors that will ultimately give rise to adult satellite cells, the stem cells responsible for muscle repair, is still largely unexplored. To investigate this aspect, we have generated a mouse strain carrying a CreER coding sequence in the Myf5 locus. In this strain, Tamoxifen-inducible Cre activity parallels endogenous Myf5 expression. Combining Myf5(CreER) and Cre reporter alleles, we were able to evaluate the contribution of cells expressing Myf5 at distinct developmental stages to the pool of satellite cells in adult hindlimb muscles. Although it was possible to trace back the origin of some rare satellite cells to a subpopulation of Myf5(+ve) progenitors in the limb buds at the late embryonic stage (∼E12), a significant number of satellite cells arise from cells which expressed Myf5 for the first time at the fetal stage (∼E15). These studies provide direct evidence that adult satellite cells derive from progenitors that first express the myogenic determination gene Myf5 during fetal stages of myogenesis.
Published by Elsevier Inc.
Figures
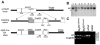
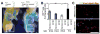



References
-
- Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp. 1983;72:163–181. - PubMed
-
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. - PubMed
-
- Biressi S, Messina G, Collombat P, Tagliafico E, Monteverde S, Benedetti L, Cusella De Angelis MG, Mansouri A, Ferrari S, Tajbakhsh S, Broccoli V, Cossu G. The homeobox gene Arx is a novel positive regulator of embryonic myogenesis. Cell Death Differ. 2008;15:94–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

