Cicletanine stimulates eNOS phosphorylation and NO production via Akt and MAP kinase/Erk signaling in sinusoidal endothelial cells
- PMID: 23639812
- PMCID: PMC3725686
- DOI: 10.1152/ajpgi.00003.2013
Cicletanine stimulates eNOS phosphorylation and NO production via Akt and MAP kinase/Erk signaling in sinusoidal endothelial cells
Erratum in
-
Corrigendum for Liu et al., volume 305, 2013, p. G163-G171.Am J Physiol Gastrointest Liver Physiol. 2022 Apr 1;322(4):G457-G458. doi: 10.1152/ajpgi.00003.2013_COR. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 35315727 Free PMC article. No abstract available.
Abstract
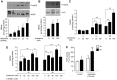
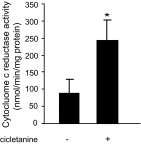
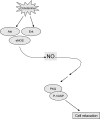
The function of the endothelial isoform of nitric oxide synthase (eNOS) and production of nitric oxide (NO) is altered in a number of disease states. Pharmacological approaches to enhancing NO synthesis and thus perhaps endothelial function could have substantial benefits in patients. We analyzed the effect of cicletanine, a synthetic pyridine with potent vasodilatory characteristics, on eNOS function and NO production in normal (liver) and injured rat sinusoidal endothelial cells, and we studied the effect of cicletanine-induced NO on stellate cell contraction and portal pressure in an in vivo model of liver injury. Sinusoidal endothelial cells were isolated from normal and injured rat livers. After exposure to cicletanine, eNOS phosphorylation, NO synthesis, and the signaling pathway regulating eNOS activation were measured. Cicletanine led to an increase in eNOS (Ser¹¹⁷⁷) phosphorylation, cytochrome c reductase activity, L-arginine conversion to L-citrulline, as well as NO production. The mechanism of the effect of cicletanine appeared to be via the protein kinase B (Akt) and MAP kinase/Erk signaling pathways. Additionally, cicletanine improved NO synthesis in injured sinusoidal endothelial cells. NO production induced by cicletanine in sinusoidal endothelial cells increased protein kinase G (PKG) activity as well as relaxation of stellate cells. Finally, administration of cicletanine to mice with portal hypertension induced by bile duct ligation led to reduction of portal pressure. The data indicate that cicletanine might improve eNOS activity in injured sinusoidal endothelial cells and likely activates hepatic stellate cell NO/PKG signaling. It raises the possibility that cicletanine could improve intrahepatic vascular function in portal hypertensive patients.
Keywords: endothelial isoform of nitric oxide synthase; extracellular/signal-regulated kinase; mitogen-activated protein kinase; portal hypertension; protein kinase B; signaling; stellate cell.
Figures






Similar articles
-
Integrin-linked kinase regulates endothelial cell nitric oxide synthase expression in hepatic sinusoidal endothelial cells.Liver Int. 2015 Apr;35(4):1213-21. doi: 10.1111/liv.12606. Epub 2014 Jul 5. Liver Int. 2015. PMID: 24906011 Free PMC article.
-
The vasoprotective effect of JP05 through the activation of PI3K/Akt-dependent eNOS and MEK/ERK pathways in brain endothelial cells.J Ethnopharmacol. 2010 Aug 9;130(3):607-13. doi: 10.1016/j.jep.2010.05.050. Epub 2010 Jun 1. J Ethnopharmacol. 2010. PMID: 20561929
-
A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension.Nat Med. 2005 Sep;11(9):952-8. doi: 10.1038/nm1289. Epub 2005 Sep 4. Nat Med. 2005. PMID: 16142243
-
A novel insight into the mechanism of pulmonary hypertension involving caveolin-1 deficiency and endothelial nitric oxide synthase activation.Trends Cardiovasc Med. 2009 Oct;19(7):238-42. doi: 10.1016/j.tcm.2010.02.003. Trends Cardiovasc Med. 2009. PMID: 20382348 Free PMC article. Review.
-
Signal transduction of platelet-induced liver regeneration and decrease of liver fibrosis.Int J Mol Sci. 2014 Mar 28;15(4):5412-25. doi: 10.3390/ijms15045412. Int J Mol Sci. 2014. PMID: 24686514 Free PMC article. Review.
Cited by
-
Adrenocorticotropic Hormone and PI3K/Akt Inhibition Reduce eNOS Phosphorylation and Increase Cortisol Biosynthesis in Long-Term Hypoxic Ovine Fetal Adrenal Cortical Cells.Reprod Sci. 2015 Aug;22(8):932-41. doi: 10.1177/1933719115570899. Epub 2015 Feb 5. Reprod Sci. 2015. PMID: 25656500 Free PMC article.
-
β-PIX cooperates with GIT1 to regulate endothelial nitric oxide synthase in sinusoidal endothelial cells.Am J Physiol Gastrointest Liver Physiol. 2022 Nov 1;323(5):G511-G522. doi: 10.1152/ajpgi.00034.2022. Epub 2022 Aug 31. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 36044673 Free PMC article.
-
Liquid plasma promotes angiogenesis through upregulation of endothelial nitric oxide synthase-induced extracellular matrix metabolism: potential applications of liquid plasma for vascular injuries.Cell Commun Signal. 2024 Feb 19;22(1):138. doi: 10.1186/s12964-023-01412-w. Cell Commun Signal. 2024. PMID: 38374138 Free PMC article.
-
Glytan decreases portal pressure via mesentery vasoconstriction in portal hypertensive rats.World J Gastroenterol. 2014 Nov 28;20(44):16674-82. doi: 10.3748/wjg.v20.i44.16674. World J Gastroenterol. 2014. PMID: 25469036 Free PMC article.
-
Thalidomide ameliorates portal hypertension via nitric oxide synthase independent reduced systolic blood pressure.World J Gastroenterol. 2015 Apr 14;21(14):4126-35. doi: 10.3748/wjg.v21.i14.4126. World J Gastroenterol. 2015. PMID: 25892862 Free PMC article.
References
-
- Akamatsu N, Sawada S, Komatsu S, Tamagaki T, Hiranuma O, Kawahara T, Tsuda Y, Kono Y, Higaki T, Tada Y, Yamasaki S, Imamura H, Sato T, Tsuji H, Nakagawa M. Effect of cicletanine on the nitric oxide pathway in human umbilical vein endothelial cells. J Cardiovasc Pharmacol 38: 174–182, 2001 - PubMed
-
- Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem 275: 30707–30715, 2000 - PubMed
-
- Bukoski RD, Bo J, Xue H, Bian K. Antiproliferative and endothelium-dependent vasodilator properties of 1,3-dihydro-3-p-chlorophenyl-7-hydroxy-6-methyl-furo-(3,4c) pyridine hydrochloride (cicletanine). J Pharmacol Exp Ther 265: 30–35, 1993 - PubMed
-
- Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. J Biol Chem 268: 8410–8413, 1993 - PubMed
-
- Calder JA, Schachter M, Sever PS. Interaction between cicletanine and the eicosanoid system in human subcutaneous resistance arteries. J Pharm Pharmacol 44: 574–578, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

