Enteric nervous system development: migration, differentiation, and disease
- PMID: 23639815
- PMCID: PMC3725693
- DOI: 10.1152/ajpgi.00452.2012
Enteric nervous system development: migration, differentiation, and disease
Abstract
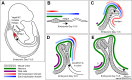
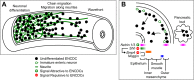
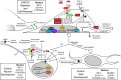
The enteric nervous system (ENS) provides the intrinsic innervation of the bowel and is the most neurochemically diverse branch of the peripheral nervous system, consisting of two layers of ganglia and fibers encircling the gastrointestinal tract. The ENS is vital for life and is capable of autonomous regulation of motility and secretion. Developmental studies in model organisms and genetic studies of the most common congenital disease of the ENS, Hirschsprung disease, have provided a detailed understanding of ENS development. The ENS originates in the neural crest, mostly from the vagal levels of the neuraxis, which invades, proliferates, and migrates within the intestinal wall until the entire bowel is colonized with enteric neural crest-derived cells (ENCDCs). After initial migration, the ENS develops further by responding to guidance factors and morphogens that pattern the bowel concentrically, differentiating into glia and neuronal subtypes and wiring together to form a functional nervous system. Molecules controlling this process, including glial cell line-derived neurotrophic factor and its receptor RET, endothelin (ET)-3 and its receptor endothelin receptor type B, and transcription factors such as SOX10 and PHOX2B, are required for ENS development in humans. Important areas of active investigation include mechanisms that guide ENCDC migration, the role and signals downstream of endothelin receptor type B, and control of differentiation, neurochemical coding, and axonal targeting. Recent work also focuses on disease treatment by exploring the natural role of ENS stem cells and investigating potential therapeutic uses. Disease prevention may also be possible by modifying the fetal microenvironment to reduce the penetrance of Hirschsprung disease-causing mutations.
Keywords: Hirschsprung disease; axonal targeting; cell migration; chain migration; development; enteric nervous system; gene-environment interactions; genetic interactions; neural crest; neural crest-derived stem cells; neurochemical coding; pseudoobstruction.
Figures
References
-
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45: 1–14, 2008 - PubMed
-
- Anderson R, Stewart A, Young H. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res 323: 11–25, 2006 - PubMed
-
- Anderson RB, Bergner AJ, Taniguchi M, Fujisawa H, Forrai A, Robb L, Young HM. Effects of different regions of the developing gut on the migration of enteric neural crest-derived cells: a role for Sema3A, but not Sema3F. Dev Biol 305: 287–299, 2007 - PubMed
-
- Anderson RB, Turner KN, Nikonenko AG, Hemperly J, Schachner M, Young HM. The cell adhesion molecule L1 is required for chain migration of neural crest cells in the developing mouse gut. Gastroenterology 130: 1221–1232, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

