"Selfish spermatogonial selection": a novel mechanism for the association between advanced paternal age and neurodevelopmental disorders
- PMID: 23639989
- PMCID: PMC4001324
- DOI: 10.1176/appi.ajp.2013.12101352
"Selfish spermatogonial selection": a novel mechanism for the association between advanced paternal age and neurodevelopmental disorders
Abstract
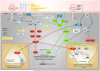
There is robust evidence from epidemiological studies that the offspring of older fathers have an increased risk of neurodevelopmental disorders, such as schizophrenia and autism. The authors present a novel mechanism that may contribute to this association. Because the male germ cell undergoes many more cell divisions across the reproductive age range, copy errors taking place in the paternal germline are associated with de novo mutations in the offspring of older men. Recently it has been recognized that somatic mutations in male germ cells that modify proliferation through dysregulation of the RAS protein pathway can lead to within-testis expansion of mutant clonal lines. First identified in association with rare disorders related to paternal age (e.g., Apert syndrome, achondroplasia), this process is known as "selfish spermatogonial selection." This mechanism favors propagation of germ cells carrying pathogenic mutations, increasingly skews the mutational profile of sperm as men age, and enriches de novo mutations in the offspring of older fathers that preferentially affect specific cellular signaling pathways. This mechanism not only offers a parsimonious explanation for the association between advanced paternal age and various neurodevelopmental disorders but also provides insights into the genetic architecture (role of de novo mutations), neurobiological correlates (altered cell cycle), and some epidemiological features of these disorders. The authors outline hypotheses to test this model. Given the secular changes for delayed parenthood in most societies, this hypothesis has important public health implications.
Figures

References
-
- Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1(1):40–7. - PubMed
-
- Crow JF. A new study challenges the current belief of a high human male:female mutation ratio. Trends Genet. 2000;16(12):525–6. - PubMed
-
- Hehir-Kwa JY, Rodriguez-Santiago B, Vissers LE, de Leeuw N, Pfundt R, Buitelaar JK, Perez-Jurado LA, Veltman JA. De novo copy number variants associated with intellectual disability have a paternal origin and age bias. J Med Genet. 2011;48(11):776–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

