Adapting smoking cessation treatment according to initial response to precessation nicotine patch
- PMID: 23640009
- PMCID: PMC4562286
- DOI: 10.1176/appi.ajp.2013.12070919
Adapting smoking cessation treatment according to initial response to precessation nicotine patch
Abstract
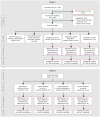
OBJECTIVE The authors evaluated an adaptive smoking cessation treatment strategy in which nicotine patch treatment was initiated before a quit date, and then, depending on initial therapeutic response, either the nicotine patch was continued or alternative pharmacotherapies were provided. METHOD The study was a double-blind, parallel-arm adaptive treatment trial. A total of 606 cigarette smokers started open-label nicotine patch treatment 2 weeks before the quit date. Those whose ad lib smoking did not decrease by >50% after 1 week were randomly assigned to one of three double-blind treatments: nicotine patch alone (control condition); "rescue" treatment with bupropion augmentation of the patch; or rescue treatment with varenicline alone. Participants whose precessation smoking decreased >50% but who lapsed after the quit date were also randomly assigned to the two rescue treatments or to nicotine patch alone. Logistic regression analyses compared each rescue treatment against the control condition in terms of abstinence at the end of treatment (weeks 8-11) and at 6 months. RESULTS Smokers who did not respond adequately to precessation nicotine patch benefited from bupropion augmentation; abstinence rates at end of treatment were 16% with nicotine patch alone and 28% with bupropion augmentation (odds ratio=2.04, 95% CI=1.03-4.01). Switching to varenicline produced less robust effects, but point abstinence at 6 months was 6.6% with the patch alone and 16.5% with a switch to varenicline (odds ratio=2.80, 95% CI=1.11-7.06). Postquit adaptive changes in treatment had no significant effects on any abstinence outcome. CONCLUSIONS It is possible to rescue a significant portion of smokers who would have failed to achieve abstinence if left on nicotine patch alone by identifying these smokers before their quit date and implementing adaptive changes in treatment.
Trial registration: ClinicalTrials.gov NCT00894166.
Figures
Comment in
-
Searching for more effective smoking cessation treatment.Am J Psychiatry. 2013 Aug;170(8):818-20. doi: 10.1176/appi.ajp.2013.13060758. Am J Psychiatry. 2013. PMID: 23903328 No abstract available.
References
-
- Bittoun R. A combination nicotine replacement therapy (NRT) algorithm for hard-to-treat smokers. J Smok Cessat. 2006;1:3–6.
-
- Fiore MC. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care. 2000;45:1196–1199. - PubMed
-
- Boshier A, Wilton LV, Shakir SA. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. Eur J Clin Pharmacol. 2003;59:767–773. - PubMed
-
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

