IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke
- PMID: 23640015
- PMCID: PMC3737266
- DOI: 10.1007/s11011-013-9413-3
IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke
Abstract
Clinical stroke induces inflammatory processes leading to cerebral injury. IL-10 expression is elevated during major CNS diseases and limits inflammation in the brain. Recent evidence demonstrated that absence of B-cells led to larger infarct volumes and increased numbers of activated T-cells, monocytes and microglial cells in the brain, thus implicating a regulatory role of B-cell subpopulations in limiting CNS damage from stroke. The aim of this study was to determine whether the IL-10-producing regulatory B-cell subset can limit CNS inflammation and reduce infarct volume following ischemic stroke in B-cell deficient (μMT(-/-)) mice. Five million IL-10-producing B-cells were obtained from IL-10-GFP reporter mice and transferred i.v. to μMT(-/-)mice. After 24 h following this transfer, recipients were subjected to 60 min of middle cerebral artery occlusion (MCAO) followed by 48 h of reperfusion. Compared to vehicle-treated controls, the IL-10(+) B-cell-replenished μMT(-/-)mice had reduced infarct volume and fewer infiltrating activated T-cells and monocytes in the affected brain hemisphere. These effects in CNS were accompanied by significant increases in regulatory T-cells and expression of the co-inhibitory receptor, PD-1, with a significant reduction in the proinflammatory milieu in the periphery. These novel observations provide the first proof of both immunoregulatory and protective functions of IL-10-secreting B-cells in MCAO that potentially could impart significant benefit for stroke patients in the clinic.
Conflict of interest statement
Figures
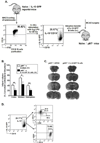
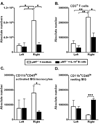

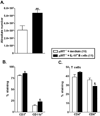
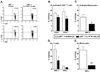

References
-
- Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. - PubMed
-
- Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, Tress BM, Colman PG, Jerums G, Chambers BR, Davis SM. The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci. 2002;9:618–626. - PubMed
-
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18(Suppl 2):s18–s22. - PubMed
-
- Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

