Glucocorticoid induction of occludin expression and endothelial barrier requires transcription factor p54 NONO
- PMID: 23640037
- PMCID: PMC3681474
- DOI: 10.1167/iovs.13-11980
Glucocorticoid induction of occludin expression and endothelial barrier requires transcription factor p54 NONO
Abstract
Purpose: Glucocorticoids (GCs) effectively reduce retinal edema and induce vascular barrier properties but possess unwanted side effects. Understanding GC induction of barrier properties may lead to more effective and specific therapies. Previous work identified the occludin enhancer element (OEE) as a GC-responsive cis-element in the promoters of multiple junctional genes, including occludin, claudin-5, and cadherin-9. Here, we identify two OEE-binding factors and determine their contribution to GC induction of tight junction (TJ) gene expression and endothelial barrier properties.
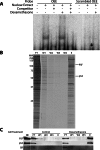
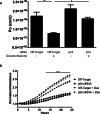
Methods: OEE-binding factors were isolated from human retinal endothelial cells (HREC) using DNA affinity purification followed by MALDI-TOF MS/MS. Chromatin immunoprecipitation (ChIP) assays determined in situ binding. siRNA was used to evaluate the role of trans-acting factors in transcription of TJ genes in response to GC stimulation. Paracellular permeability was determined by quantifying flux through a cell monolayer, whereas transendothelial electrical resistance (TER) was measured using the ECIS system.
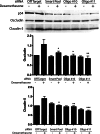
Results: MS/MS analysis of HREC nuclear extracts identified the heterodimer of transcription factors p54/NONO (p54) and polypyrimidine tract-binding protein-associated splicing factor (PSF) as OEE-binding factors, which was confirmed by ChIP assay from GC-treated endothelial cells and rat retina. siRNA knockdown of p54 demonstrated that this factor is necessary for GC induction of occludin and claudin-5 expression. Further, p54 knockdown ablated the pro-barrier effects of GC treatment.
Conclusions: p54 is essential for GC-mediated expression of occludin, claudin-5, and barrier induction, and the p54/PSF heterodimer may contribute to normal blood-retinal barrier (BRB) induction in vivo. Understanding the mechanism of GC induction of BRB properties may provide novel therapies for macular edema.
Keywords: blood-retinal barrier; enhancer; gene expression; tight junction.
Figures


Comment in
-
A novel mechanism for glucocorticoid-induced tightening of endothelial barriers.Invest Ophthalmol Vis Sci. 2013 Jun 12;54(6):4016. doi: 10.1167/iovs.13-12400. Invest Ophthalmol Vis Sci. 2013. PMID: 23759428 No abstract available.
Similar articles
-
Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element.Exp Eye Res. 2008 Jun;86(6):867-78. doi: 10.1016/j.exer.2008.01.002. Epub 2008 Jan 12. Exp Eye Res. 2008. PMID: 18501346 Free PMC article.
-
A novel mechanism for glucocorticoid-induced tightening of endothelial barriers.Invest Ophthalmol Vis Sci. 2013 Jun 12;54(6):4016. doi: 10.1167/iovs.13-12400. Invest Ophthalmol Vis Sci. 2013. PMID: 23759428 No abstract available.
-
p54nrb/NONO regulates cyclic AMP-dependent glucocorticoid production by modulating phosphodiesterase mRNA splicing and degradation.Mol Cell Biol. 2015 Apr;35(7):1223-37. doi: 10.1128/MCB.00993-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605330 Free PMC article.
-
Glucocorticoids exert differential effects on the endothelium in an in vitro model of the blood-retinal barrier.Acta Ophthalmol. 2019 Mar;97(2):214-224. doi: 10.1111/aos.13909. Epub 2018 Aug 31. Acta Ophthalmol. 2019. PMID: 30168271
-
Glucocorticoids in the management of peritumoral brain edema: a review of molecular mechanisms.Childs Nerv Syst. 2016 Dec;32(12):2293-2302. doi: 10.1007/s00381-016-3240-x. Epub 2016 Sep 9. Childs Nerv Syst. 2016. PMID: 27613642 Free PMC article. Review.
Cited by
-
Identification of genes and transcription factors associated with glucocorticoid response in lens epithelial cells.Mol Med Rep. 2015 Jun;11(6):4073-8. doi: 10.3892/mmr.2015.3308. Epub 2015 Feb 6. Mol Med Rep. 2015. PMID: 25672806 Free PMC article.
-
Endothelial Barrier Integrity Is Disrupted In Vitro by Heme and by Serum From Sickle Cell Disease Patients.Front Immunol. 2020 Dec 14;11:535147. doi: 10.3389/fimmu.2020.535147. eCollection 2020. Front Immunol. 2020. PMID: 33381108 Free PMC article.
-
Diabetic macular edema: new concepts in patho-physiology and treatment.Cell Biosci. 2014 May 14;4:27. doi: 10.1186/2045-3701-4-27. eCollection 2014. Cell Biosci. 2014. PMID: 24955234 Free PMC article. Review.
-
Pharmacology of Corticosteroids for Diabetic Macular Edema.Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):1-12. doi: 10.1167/iovs.17-22259. Invest Ophthalmol Vis Sci. 2018. PMID: 29297055 Free PMC article. Review.
-
LncRNA NEAT1 Recruits SFPQ to Regulate MITF Splicing and Control RPE Cell Proliferation.Invest Ophthalmol Vis Sci. 2021 Nov 1;62(14):18. doi: 10.1167/iovs.62.14.18. Invest Ophthalmol Vis Sci. 2021. PMID: 34787639 Free PMC article.
References
-
- Klein R, Klein BE, Moss SE. The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Metab Rev. 1989; 5: 559–570 - PubMed
-
- Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012; 12: CD007419 - PubMed
-
- Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012; 130: 972–979 - PubMed
-
- Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E. The use of intravitreal etanercept in diabetic macular oedema. Semin Ophthalmol. 2007; 22: 75–79 - PubMed
-
- Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012; 119: 2125–2132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

