Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9
- PMID: 23640040
- PMCID: PMC5110072
- DOI: 10.1167/iovs.12-10816
Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9
Abstract
Purpose: Nerve growth factor (NGF) is a neuropeptide essential for the development, survival, growth, and differentiation of corneal cells. Its effects are mediated by both TrkA and p75 receptors. Clinically relevant use of NGF was introduced to treat neurotrophic ulcerations in patients. Herein, we examine the mechanisms by which NGF enhances epithelial wound healing both in vivo and in vitro.
Methods: An animal model using adult hens was implemented for the in vivo experiments. Laser ablation keratectomy was performed and animals were observed for up to 7 days. Epithelial healing was measured with fluorescein. In addition, proliferation was measured using BrdU incorporation and both TrkA and matrix metalloprotease-9 (MMP-9) expression were measured by immunohistochemistry (IHC) and Western blot (WB). In vitro experiments were carried out with telomerase-immortalized human corneal epithelial cells (HCLE). The rate of proliferation was measured using a colorimetric assay and BrdU incorporation. Real-time migration was evaluated with an inverted microscope. MMP-9 expression was evaluated by immunocytochemistry (ICC), WB, zymography, and RT-PCR. Finally, beta-4 integrin (β4) expression was assessed by ICC and WB.
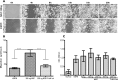
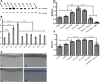
Results: Faster epithelial healing was observed in NGF-treated corneas compared with controls (P < 0.01). These corneas showed increased proliferation, TrkA upregulation, and enhanced MMP-9 presence (P < 0.01). In vitro, faster spreading and migration were observed in response to NGF (P < 0.01). Enhanced proliferation, as well as enhanced TrkA and MMP-9 expression, and decreased β4 levels were observed after adding NGF (P < 0.01).
Conclusions: NGF plays a major role during the epithelial healing process by promoting migration, a process that is accelerated by cell spreading. This effect is mediated by both the upregulation of MMP-9 and cleavage of β4 integrin.
Keywords: MMP-9; NGF; TrkA; corneal epithelium migration; corneal epithelium proliferation.
Figures




References
-
- Kawamoto K, Matsuda H. Nerve growth factor and wound healing. Prog Brain Res. 2004; 146: 369–384. - PubMed
-
- Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000; 107: 1347–1351; discussion 1351–1352. - PubMed
-
- Lambiase A, Manni L, Rama P, Bonini S. Clinical application of nerve growth factor on human corneal ulcer. Arch Ital Biol. 2003; 141: 141–148. - PubMed
-
- Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006; 25: 352–355. - PubMed
-
- Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008; 57: 253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

