Caffeic acid phenethyl ester suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway
- PMID: 23640046
- PMCID: PMC3765043
- DOI: 10.1093/carcin/bgt154
Caffeic acid phenethyl ester suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway
Abstract
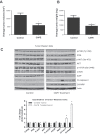
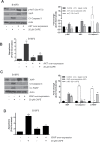
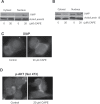
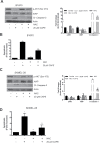
Melanoma is highly metastatic and resistant to chemotherapeutic drugs. Our previous studies have demonstrated that caffeic acid phenethyl ester (CAPE) suppresses the growth of melanoma cells and induces reactive oxygen species generation. However, the exact mechanism of the growth suppressive effects of CAPE was not clear. Here, we determined the potential mechanism of CAPE against melanoma in vivo and in vitro. Administration of 10 mg/kg/day CAPE substantially suppressed the growth of B16F0 tumor xenografts in C57BL/6 mice. Tumors from CAPE-treated mice showed reduced phosphorylation of phosphoinositide 3-kinase, AKT, mammalian target of rapamycin and protein level of X-linked inhibitor of apoptosis protein (XIAP) and enhanced the cleavage of caspase-3 and poly (ADP ribose) polymerase. In order to confirm the in vivo observations, melanoma cells were treated with CAPE. CAPE treatment suppressed the activating phosphorylation of phosphoinositide 3-kinase at Tyr 458, phosphoinositide-dependent kinase-1 at Ser 241, mammalian target of rapamycin at Ser 2448 and AKT at Ser 473 in B16F0 and SK-MEL-28 cells in a concentration and time-dependent study. Furthermore, the expression of XIAP, survivin and BCL-2 was downregulated by CAPE treatment in both cell lines. Significant apoptosis was observed by CAPE treatment as indicated by cleavage of caspase-3 and poly (ADP ribose) polymerase. AKT kinase activity was inhibited by CAPE in a concentration-dependent manner. CAPE treatment increased the nuclear translocation of XIAP, indicating increased apoptosis in melanoma cells. To confirm the involvement of reactive oxygen species in the inhibition of AKT/XIAP pathway, cells were treated with antioxidant N-acetyl-cysteine (NAC) prior to CAPE treatment. Our results indicate that NAC blocked CAPE-mediated AKT/XIAP inhibition and protected the cells from apoptosis. Because AKT regulates XIAP, their interaction was examined by immunoprecipitation studies. Our results show that CAPE treatment decreased the interaction of AKT with XIAP. To establish the involvement of AKT in the apoptosis-inducing effects of CAPE, cells were transfected with AKT. Our results revealed that AKT overexpression attenuated the decrease in XIAP and significantly blocked CAPE-mediated apoptosis. Similarly, overexpression of XIAP further decreased CAPE-induced apoptosis. Taken together, our results suggest that CAPE suppresses phosphoinositide 3-kinase/AKT/XIAP pathway leading to apoptosis in melanoma tumor cells in vitro and in vivo.
Figures


Similar articles
-
Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks.Cancer Prev Res (Phila). 2012 May;5(5):788-97. doi: 10.1158/1940-6207.CAPR-12-0004-T. Cancer Prev Res (Phila). 2012. PMID: 22562408 Free PMC article.
-
Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatory responses in human gingival fibroblasts via NF-κB and PI3K/Akt signaling pathway.Eur J Pharmacol. 2017 Jan 5;794:61-68. doi: 10.1016/j.ejphar.2016.11.003. Epub 2016 Nov 7. Eur J Pharmacol. 2017. PMID: 27832944
-
Targeting of X-linked inhibitor of apoptosis protein and PI3-kinase/AKT signaling by embelin suppresses growth of leukemic cells.PLoS One. 2017 Jul 13;12(7):e0180895. doi: 10.1371/journal.pone.0180895. eCollection 2017. PLoS One. 2017. PMID: 28704451 Free PMC article.
-
The critical role of X-linked inhibitor of apoptosis protein (XIAP) in tumor development.Apoptosis. 2025 Jun;30(5-6):1202-1215. doi: 10.1007/s10495-025-02101-4. Epub 2025 Mar 27. Apoptosis. 2025. PMID: 40146486 Review.
-
Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer.Pharmacol Res. 2021 Sep;171:105759. doi: 10.1016/j.phrs.2021.105759. Epub 2021 Jul 8. Pharmacol Res. 2021. PMID: 34245864 Review.
Cited by
-
Phenolic Compounds Contribution to Portuguese Propolis Anti-Melanoma Activity.Molecules. 2023 Mar 30;28(7):3107. doi: 10.3390/molecules28073107. Molecules. 2023. PMID: 37049869 Free PMC article.
-
Inhibiting CDK6 Activity by Quercetin Is an Attractive Strategy for Cancer Therapy.ACS Omega. 2020 Oct 14;5(42):27480-27491. doi: 10.1021/acsomega.0c03975. eCollection 2020 Oct 27. ACS Omega. 2020. PMID: 33134711 Free PMC article.
-
Evaluation of the In Vitro Cytotoxic Activity of Caffeic Acid Derivatives and Liposomal Formulation against Pancreatic Cancer Cell Lines.Materials (Basel). 2020 Dec 19;13(24):5813. doi: 10.3390/ma13245813. Materials (Basel). 2020. PMID: 33352809 Free PMC article.
-
Incaspitolide A isolated from Carpesium cernuum L. inhibits the growth of prostate cancer cells and induces apoptosis via regulation of the PI3K/Akt/xIAP pathway.Oncol Lett. 2021 Jun;21(6):477. doi: 10.3892/ol.2021.12738. Epub 2021 Apr 19. Oncol Lett. 2021. PMID: 33968193 Free PMC article.
-
The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products.Antioxidants (Basel). 2022 Sep 19;11(9):1845. doi: 10.3390/antiox11091845. Antioxidants (Basel). 2022. PMID: 36139919 Free PMC article. Review.
References
-
- Kudugunti S.K., et al. (2011). Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Invest. New Drugs 29, 52–62 - PubMed
-
- Cartee T.V., et al. (2011). Melanoma reporting to central cancer registries by US dermatologists: an analysis of the persistent knowledge and practice gap. J. Am. Acad. Dermatol., 65(5 suppl. 1),S124–S132 - PubMed
-
- Doan P.S. (2008). Primary cutaneous malignant melanoma. Aviat. Space. Environ. Med., 79, 919–921 - PubMed
-
- Watson M., et al. (2011). Melanoma surveillance in the United States: overview of methods. J. Am. Acad. Dermatol., 65(5 suppl. 1), S6–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

