Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells
- PMID: 23640167
- PMCID: PMC3927835
- DOI: 10.1038/nprot.2013.049
Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells
Abstract
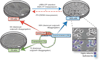
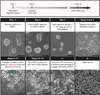
At the time of implantation in the maternal uterus, the mouse blastocyst possesses an inner cell mass comprising two lineages: epiblast (Epi) and primitive endoderm (PrE). Representative stem cells derived from these two cell lineages can be expanded and maintained indefinitely in vitro as either embryonic stem (ES) or XEN cells, respectively. Here we describe protocols that can be used to establish XEN cell lines. These include the establishment of XEN cells from blastocyst-stage embryos in either standard embryonic or trophoblast stem (TS) cell culture conditions. We also describe protocols for establishing XEN cells directly from ES cells by either retinoic acid and activin-based conversion or by overexpression of the GATA transcription factor Gata6. XEN cells are a useful model of PrE cells, with which they share gene expression, differentiation potential and lineage restriction. The robust protocols for deriving XEN cells described here can be completed within 2-3 weeks.
Figures



References
-
- Schrode N, et al. Anatomy of a blastocyst: cell behaviors driving cell fate choice and morphogenesis in the early mouse embryo. Genesis. Published online; http://dx.doi.org/10.1002/dvg.22368 (25 February 2013). - DOI - PMC - PubMed
-
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. - PubMed
-
- Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. - PubMed
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

