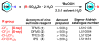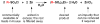Preparation and purification of zinc sulfinate reagents for drug discovery
- PMID: 23640168
- PMCID: PMC4114315
- DOI: 10.1038/nprot.2013.059
Preparation and purification of zinc sulfinate reagents for drug discovery
Abstract
The present protocol details the synthesis of zinc bis(alkanesulfinate)s that can be used as general reagents for the formation of radical species. The zinc sulfinates described herein are generated from the corresponding sulfonyl chlorides by treatment with zinc dust. The products may be used crude, or a simple purification procedure may be performed to minimize incorporation of water and zinc chloride. Although the synthesis of the zinc sulfinate salts can generally be completed within 3 h, workup can take up to 24 h and purification can take up to 3 h. Following the steps in this protocol would enable the user to generate a small toolkit of zinc sulfinate reagents over the course of 1 week.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Weidner JP, Block SS. Infrared spectra of zinc sulfinates. Appl Spectroscopy. 1969;23:337–341.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous