Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice
- PMID: 23640395
- PMCID: PMC3681012
- DOI: 10.1038/bjc.2013.206
Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice
Abstract
Background: Myeloid-derived suppressor cells (MDSCs) are present in large numbers in blood of mice and humans with cancer, and they strongly inhibit T-cell and natural killer (NK) cell responses, at young and old age. We found that a highly attenuated bacterium Listeria monocytogenes (Listeria(at))-infected MDSC and altered the immune-suppressing function of MDSC.
Methods: Young (3 months) and old (18 months) BALB/cByJ mice with metastatic breast cancer (4T1 model) were immunised with Listeria(at) semi-therapeutically (once before and twice after tumour development), and analysed for growth of metastases and primary tumour, in relation to MDSC-, CD8 T-cell and NK cell responses.
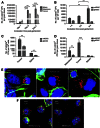
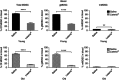
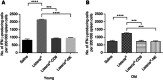
Results: We found that Listeria(at)-infected MDSC, which delivered Listeria(at) predominantly to the microenvironment of metastases and primary tumours, where they spread from MDSC into tumour cells (infected tumour cells will ultimately become a target for Listeria-activated immune cells). Immunotherapy with Listeria(at) significantly reduced the population of MDSC in blood and primary tumours, and converted a remaining subpopulation of MDSC into an immune-stimulating phenotype producing IL-12, in correlation with significantly improved T-cell and NK cell responses to Listeria(at) at both ages. This was accompanied with a dramatic reduction in the number of metastases and tumour growth at young and old age.
Conclusions: Although preclinical studies show that immunotherapy is less effective at old than at young age, our study demonstrates that Listeria(at)-based immunotherapy can be equally effective against metastatic breast cancer at both young and old age by targeting MDSC.
Figures



References
-
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52 (6:1399–1405. - PubMed
-
- De Backer O, Verheyden AM, Martin B, Godelaine D, De Plaen E, Brasseur R, Avner P, Boon T. Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics. 1995;28 (1:74–83. - PubMed
-
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58 (1:49–59. - PMC - PubMed
-
- Extermann M. Basic assessment of the older cancer patient. Curr Treat Options Oncol. 2011;12 (3:276–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

