p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear
- PMID: 23640496
- PMCID: PMC3938029
- DOI: 10.1161/ATVBAHA.112.301165
p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear
Abstract
Objective: Rho GTPase proteins play a central role in regulating the dynamics of the platelet actin cytoskeleton. Yet, little is known regarding how Rho GTPase activation coordinates platelet activation and function. In this study, we aimed to characterize the role of the Rho GTPase effector, p21 activated kinase (PAK), in platelet activation, lamellipodia formation, and aggregate formation under shear.
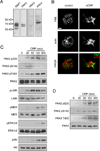
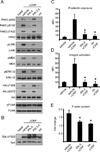
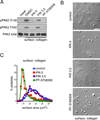
Approach and results: Stimulation of platelets with the glycoprotein receptor VI agonist, collagen-related peptide, rapidly activated PAK in a time course preceding phosphorylation of PAK substrates, LIM domain kinase LIMK1 and the MAPK/ERK kinase MEK, and the subsequent activation of MAPKs and Akt. Pharmacological inhibitors of PAK blocked signaling events downstream of PAK and prevented platelet secretion as well as platelet aggregation in response to collagen-related peptide. PAK inhibitors also prevented PAK activation and platelet spreading on collagen surfaces. PAK was also required for the formation of platelet aggregates and to maintain aggregate stability under physiological shear flow conditions.
Conclusions: These results suggest that PAK serves as an orchestrator of platelet functional responses after activation downstream of the platelet collagen receptor, glycoprotein receptor VI.
Keywords: cell signaling; platelet; signal transduction.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

