Detection of in vivo enzyme activity with CatalyCEST MRI
- PMID: 23640714
- PMCID: PMC3742626
- DOI: 10.1002/mrm.24763
Detection of in vivo enzyme activity with CatalyCEST MRI
Abstract
Purpose: CatalyCEST MRI compares the detection of an enzyme-responsive chemical exchange saturation transfer (CEST) agent with the detection of an unresponsive "control" CEST agent that accounts for other conditions that influence CEST. The purpose of this study was to investigate the feasibility of in vivo catalyCEST MRI.
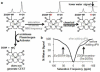
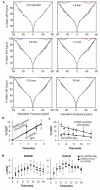
Methods: CEST agents that were responsive and unresponsive to the activity of urokinase plasminogen activator were shown to have negligible interaction with each other. A CEST-fast imaging with steady state precession (FISP) MRI protocol was used to acquire MR CEST spectroscopic images with a Capan-2 pancreatic tumor model after intravenous injection of the CEST agents. A function of (super)-Lorentzian line shapes was fit to CEST spectra of a region-of-interest that represented the tumor.
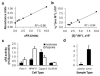
Results: The CEST effects from each agent showed the same initial uptake into tumor tissues, indicating that both agents had the same pharmacokinetic transport rates. Starting 5 min after injection, CEST from the enzyme-responsive agent disappeared more quickly than CEST from the unresponsive agent, indicating that the enzyme responsive agent was being catalyzed by urokinase plasminogen activator, while both agents also experienced net pharmacokinetic washout from the tumor.
Conclusion: CatalyCEST MRI demonstrates that dynamic tracking of enzyme-responsive and unresponsive CEST agents during the same in vivo MRI study is feasible.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
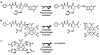


Similar articles
-
Detecting in vivo urokinase plasminogen activator activity with a catalyCEST MRI contrast agent.NMR Biomed. 2017 Jul;30(7):10.1002/nbm.3721. doi: 10.1002/nbm.3721. Epub 2017 Mar 29. NMR Biomed. 2017. PMID: 28370884 Free PMC article.
-
Noninvasive detection of enzyme activity in tumor models of human ovarian cancer using catalyCEST MRI.Magn Reson Med. 2017 May;77(5):2005-2014. doi: 10.1002/mrm.26278. Epub 2016 May 25. Magn Reson Med. 2017. PMID: 27221386 Free PMC article.
-
Ytterbium chelated to 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid,10-orthoaminoanilide.2011 Nov 26 [updated 2012 Jan 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Nov 26 [updated 2012 Jan 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22238803 Free Books & Documents. Review.
-
A single diamagnetic catalyCEST MRI contrast agent that detects cathepsin B enzyme activity by using a ratio of two CEST signals.Contrast Media Mol Imaging. 2016 Mar-Apr;11(2):130-8. doi: 10.1002/cmmi.1672. Epub 2015 Dec 3. Contrast Media Mol Imaging. 2016. PMID: 26633584 Free PMC article.
-
Assessments of tumor metabolism with CEST MRI.NMR Biomed. 2019 Oct;32(10):e3943. doi: 10.1002/nbm.3943. Epub 2018 Jun 25. NMR Biomed. 2019. PMID: 29938857 Free PMC article. Review.
Cited by
-
The role of responsive MRI probes in the past and the future of molecular imaging.Chem Sci. 2024 Nov 27;15(48):20122-20154. doi: 10.1039/d4sc04849k. eCollection 2024 Dec 11. Chem Sci. 2024. PMID: 39611034 Free PMC article. Review.
-
Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine.Dis Model Mech. 2015 Apr;8(4):323-36. doi: 10.1242/dmm.018499. Epub 2015 Apr 1. Dis Model Mech. 2015. PMID: 26035841 Free PMC article. Review.
-
An enzyme-activatable and cell-permeable MnIII-porphyrin as a highly efficient T1 MRI contrast agent for cell labeling.Chem Sci. 2016 Jul 1;7(7):4308-4317. doi: 10.1039/c5sc04252f. Epub 2016 Mar 16. Chem Sci. 2016. PMID: 30155077 Free PMC article.
-
A CatalyCEST MRI Contrast Agent that Can Simultaneously Detect Two Enzyme Activities.Chembiochem. 2016 Mar 2;17(5):383-7. doi: 10.1002/cbic.201500586. Epub 2016 Feb 3. Chembiochem. 2016. PMID: 26693680 Free PMC article.
-
Lanthanide DO3A-Complexes Bearing Peptide Substrates: The Effect of Peptidic Side Chains on Metal Coordination and Relaxivity.Molecules. 2021 Apr 9;26(8):2176. doi: 10.3390/molecules26082176. Molecules. 2021. PMID: 33918899 Free PMC article.
References
-
- Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996;2:613–618. - PubMed
-
- Koblinski JE, Ahram M, Sloane BL. Unraveling the role of proteases in cancer. Clinica Chimica Acta. 2000;291:113–135. - PubMed
-
- Nguyen DH, Hussaini IM, Gonias SL. Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Biol Chem. 1998;273:8502–8507. - PubMed
-
- Yoo B, Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Front Biosci. 2007;13:1733–1752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

