Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism
- PMID: 23640897
- PMCID: PMC3689958
- DOI: 10.1074/jbc.M112.436584
Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism
Abstract
Homeostasis is essential for cell survival. However, homeostatic regulation of surface epithelia is poorly understood. The eye surface, lacking the cornified barrier of skin, provides an excellent model. Tears cover the surface of the eye and are deficient in dry eye, the most common eye disease affecting at least 5% of the world's population. Only a tiny fraction of the tear proteome appears to be affected, including lacritin, an epithelium-selective mitogen that promotes basal tearing when topically applied to rabbit eyes. Here we show that homeostasis of cultured corneal epithelia is entirely lacritin-dependent and elucidate the mechanism as a rapid autophagic flux to promptly restore cellular metabolism and mitochondrial fusion in keeping with the short residence time of lacritin on the eye. Accelerated flux appears to be derived from lacritin-stimulated acetylation of FOXO3 as a novel ligand for ATG101 and coupling of stress-acetylated FOXO1 with ATG7 (which remains uncoupled without lacritin) and be sufficient to selectively divert huntingtin mutant Htt103Q aggregates largely without affecting non-aggregated Htt25Q. This is in keeping with stress as a prerequisite for lacritin-stimulated autophagy. Lacritin targets the cell surface proteoglycan syndecan-1 via its C-terminal amino acids Leu(108)-Leu(109)-Phe(112) and is also available in saliva, plasma, and lung lavage. Thus, lacritin may promote epithelial homeostasis widely.
Keywords: ATG101; Autophagy; Cell Metabolism; Cornea; Dry Eye; FOXO3; Lacritin; Metabolomics; Proteoglycan; Stress.
Figures
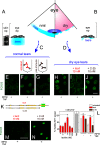
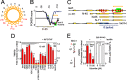


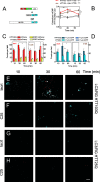
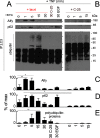
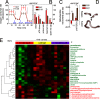

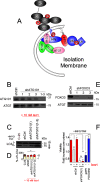
References
-
- Hotamisligil G. S. (2006) Inflammation and metabolic disorders. Nature 444, 860–867 - PubMed
-
- Lamark T., Johansen T. (2010) Autophagy: links with the proteasome. Curr. Opin. Cell Biol. 22, 192–198 - PubMed
-
- Zhao Y., Yang J., Liao W., Liu X., Zhang H., Wang S., Wang D., Feng J., Yu L., Zhu W. G. (2010) Cytosolic FOXO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 12, 665–675 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

