Diaphragm muscle remodeling in a rat model of chronic intermittent hypoxia
- PMID: 23640977
- PMCID: PMC3707358
- DOI: 10.1369/0022155413490947
Diaphragm muscle remodeling in a rat model of chronic intermittent hypoxia
Abstract
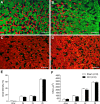
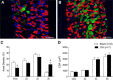
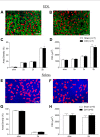
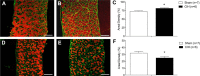
Respiratory muscle remodeling occurs in human sleep apnea--a common respiratory disorder characterized by chronic intermittent hypoxia (CIH) due to recurrent apnea during sleep. We sought to determine if CIH causes remodeling in rat sternohyoid (upper airway dilator) and diaphragm muscles. Adult male Wistar rats were exposed to CIH (n=8), consisting of 90 sec of hypoxia (5% at the nadir; SaO₂ ~80%)/90 sec of normoxia, 8 hr per day, for 7 consecutive days. Sham animals (n=8) were exposed to alternating air/air cycles in parallel. The effect of CIH on myosin heavy-chain (MHC) isoform (1, 2a, 2x, 2b) distribution, sarcoplasmic reticulum calcium ATPase (SERCA) isoform distribution, succinate dehydrogenase activity, glycerol phosphate dehydrogenase activity, and Na⁺/K⁺ ATPase pump content was determined. Sternohyoid muscle structure was unaffected by CIH treatment. CIH did not alter oxidative/glycolytic capacity or the Na⁺/K⁺-ATPase pump content of the diaphragm. CIH significantly increased the areal density of MHC 2b fibers in the rat diaphragm, and this was associated with a shift in SERCA proteins from SERCA2 to SERCA1. We conclude that CIH causes a slow-to-fast fiber transition in the rat diaphragm after just 7 days of treatment. Respiratory muscle functional remodeling may drive aberrant functional plasticity such as decreased muscle endurance, which is a feature of human sleep apnea.
Keywords: chronic intermittent hypoxia; diaphragm dysfunction; myosin heavy chain isoform distribution; obstructive sleep apnea; respiratory muscles.
Conflict of interest statement
Figures



References
-
- Allen DG, Lamb GD, Westerblad H. 2008. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 88:287–332 - PubMed
-
- Aubier M, Viires N. 1998. Calcium ATPase and respiratory muscle function. Eur Respir J. 11:758–766 - PubMed
-
- Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ. 2005. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in vitro maximal Na+-K+-ATPase activity in well-trained athletes. J Appl Physiol. 98:186–192 - PubMed
-
- Baldwin KM, Haddad F. 2002. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil. 81:S40–S51 - PubMed
-
- Bradford A. 2004. Effects of chronic intermittent asphyxia on haematocrit, pulmonary arterial pressure and skeletal muscle structure in rats. Exp Physiol. 89:44–52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

