Chloride binding site of neurotransmitter sodium symporters
- PMID: 23641004
- PMCID: PMC3666746
- DOI: 10.1073/pnas.1221279110
Chloride binding site of neurotransmitter sodium symporters
Abstract
Neurotransmitter:sodium symporters (NSSs) play a critical role in signaling by reuptake of neurotransmitters. Eukaryotic NSSs are chloride-dependent, whereas prokaryotic NSS homologs like LeuT are chloride-independent but contain an acidic residue (Glu290 in LeuT) at a site where eukaryotic NSSs have a serine. The LeuT-E290S mutant displays chloride-dependent activity. We show that, in LeuT-E290S cocrystallized with bromide or chloride, the anion is coordinated by side chain hydroxyls from Tyr47, Ser290, and Thr254 and the side chain amide of Gln250. The bound anion and the nearby sodium ion in the Na1 site organize a connection between their coordinating residues and the extracellular gate of LeuT through a continuous H-bond network. The specific insights from the structures, combined with results from substrate binding studies and molecular dynamics simulations, reveal an anion-dependent occlusion mechanism for NSS and shed light on the functional role of chloride binding.
Keywords: SLC6; X-ray crystallography; antidepressant; membrane transport; psychostimulant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
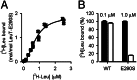

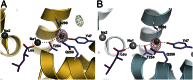

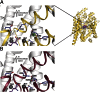
References
-
- Hediger MA, et al. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447(5):465–468. - PubMed
-
- Rudnick G. In: Mechanisms of Biogenic Amine Neurotransmitter Transporters. Reith MEA, editor. Totowa, NJ: Humana Press; 2002. pp. 25–52.
-
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: Structure, regulation and function. Nat Rev Neurosci. 2003;4(1):13–25. - PubMed
-
- Singh SK. LeuT: A prokaryotic stepping stone on the way to a eukaryotic neurotransmitter transporter structure. Channels (Austin) 2008;2(5):380–389. - PubMed
-
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: Molecular function of important drug targets. Trends Pharmacol Sci. 2006;27(7):375–383. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

