Germline deletion of Cetn1 causes infertility in male mice
- PMID: 23641067
- PMCID: PMC3711207
- DOI: 10.1242/jcs.128587
Germline deletion of Cetn1 causes infertility in male mice
Abstract
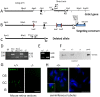
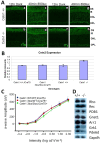
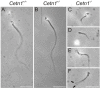
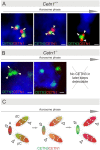
Centrins are calmodulin-like Ca(2+)-binding proteins that can be found in all ciliated eukaryotic cells from yeast to mammals. Expressed in male germ cells and photoreceptors, centrin 1 (CETN1) resides in the photoreceptor transition zone and connecting cilium. To identify its function in mammals, we deleted Cetn1 by homologous recombination. Cetn1(-/-) mice were viable and showed no sign of retina degeneration suggesting that CETN1 is nonessential for photoreceptor ciliogenesis or structural maintenance. Phototransduction components localized normally to the Cetn1(-/-) photoreceptor outer segments, and loss of CETN1 had no effect on light-induced translocation of transducin to the inner segment. Although Cetn1(-/-) females and Cetn1(+/-) males had normal fertility, Cetn1(-/-) males were infertile. The Cetn1(-/-) testes size was normal, and spermatogonia as well as spermatocytes developed normally. However, spermatids lacked tails suggesting severe defects at the late maturation phase of spermiogenesis. Viable sperm cells were absent and the few surviving spermatozoa were malformed. Light and electron microscopy analyses of Cetn1(-/-) spermatids revealed failures in centriole rearrangement during basal body maturation and in the basal-body-nucleus connection. These results confirm an essential role for CETN1 in late steps of spermiogenesis and spermatid maturation.
Keywords: CETN1 deletion; Centrin; Flagella; Photoreceptors; Spermatid maturation; Spermiogenesis.
Figures




References
-
- Avasthi P., Watt C. B., Williams D. S., Le Y. Z., Li S., Chen C. K., Marc R. E., Frederick J. M., Baehr W. (2009). Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J. Neurosci. 29, 14287–14298 10.1523/JNEUROSCI.3976-09.2009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

