Chemokines induce axon outgrowth downstream of Hepatocyte Growth Factor and TCF/β-catenin signaling
- PMID: 23641195
- PMCID: PMC3639410
- DOI: 10.3389/fncel.2013.00052
Chemokines induce axon outgrowth downstream of Hepatocyte Growth Factor and TCF/β-catenin signaling
Abstract
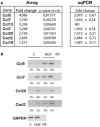
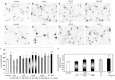
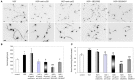
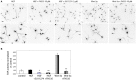
Axon morphogenesis is a complex process regulated by a variety of secreted molecules, including morphogens and growth factors, resulting in the establishment of the neuronal circuitry. Our previous work demonstrated that growth factors [Neurotrophins (NT) and Hepatocyte Growth Factor (HGF)] signal through β-catenin during axon morphogenesis. HGF signaling promotes axon outgrowth and branching by inducing β-catenin phosphorylation at Y142 and transcriptional regulation of T-Cell Factor (TCF) target genes. Here, we asked which genes are regulated by HGF signaling during axon morphogenesis. An array screening indicated that HGF signaling elevates the expression of chemokines of the CC and CXC families. In line with this, CCL7, CCL20, and CXCL2 significantly increase axon outgrowth in hippocampal neurons. Experiments using blocking antibodies and chemokine receptor antagonists demonstrate that chemokines act downstream of HGF signaling during axon morphogenesis. In addition, qPCR data demonstrates that CXCL2 and CCL5 expression is stimulated by HGF through Met/b-catenin/TCF pathway. These results identify CC family members and CXCL2 chemokines as novel regulators of axon morphogenesis downstream of HGF signaling.
Keywords: axon; beta-catenin; chemokine; hepatocyte growth factor; hippocampal neurons; neurite outgrowth.
Figures
Similar articles
-
Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation.J Cell Sci. 2008 Aug 15;121(Pt 16):2718-30. doi: 10.1242/jcs.029660. Epub 2008 Jul 29. J Cell Sci. 2008. PMID: 18664491
-
The chemokine KC regulates HGF-stimulated epithelial cell morphogenesis.Am J Physiol Renal Physiol. 2004 Mar;286(3):F581-9. doi: 10.1152/ajprenal.00289.2003. Epub 2003 Nov 4. Am J Physiol Renal Physiol. 2004. PMID: 14600031
-
Nuclear phosphorylated Y142 β-catenin accumulates in astrocytomas and glioblastomas and regulates cell invasion.Cell Cycle. 2015;14(22):3644-55. doi: 10.1080/15384101.2015.1104443. Cell Cycle. 2015. PMID: 26654598 Free PMC article.
-
Hepatocyte growth factor in lung morphogenesis and tumor invasion: role as a mediator in epithelium-mesenchyme and tumor-stroma interactions.Cancer Chemother Pharmacol. 1996;38 Suppl:S42-7. doi: 10.1007/s002800051037. Cancer Chemother Pharmacol. 1996. PMID: 8765416 Review.
-
Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know?Endocr Rev. 2005 Dec;26(7):898-915. doi: 10.1210/er.2003-0034. Epub 2005 Aug 26. Endocr Rev. 2005. PMID: 16126938 Review.
Cited by
-
Astrocytes Respond to Environment-Relevant Doses TCDD in a Specific Manner Which Is Different from the Endogenous AhR Ligand (FICZ).Environ Health (Wash). 2025 Jan 7;3(4):392-401. doi: 10.1021/envhealth.4c00189. eCollection 2025 Apr 18. Environ Health (Wash). 2025. PMID: 40270526 Free PMC article.
-
Neuronal chemokines: new insights into neuronal communication after injury.Neural Regen Res. 2023 Nov;18(11):2379-2380. doi: 10.4103/1673-5374.371352. Neural Regen Res. 2023. PMID: 37282457 Free PMC article. No abstract available.
-
CXCL1 and CXCL2 Inhibit the Axon Outgrowth in a Time- and Cell-Type-Dependent Manner in Adult Rat Dorsal Root Ganglia Neurons.Neurochem Res. 2019 Sep;44(9):2215-2229. doi: 10.1007/s11064-019-02861-x. Epub 2019 Aug 17. Neurochem Res. 2019. PMID: 31422522
-
SPARCL1 suppresses osteosarcoma metastasis and recruits macrophages by activation of canonical WNT/β-catenin signaling through stabilization of the WNT-receptor complex.Oncogene. 2018 Feb 22;37(8):1049-1061. doi: 10.1038/onc.2017.403. Epub 2017 Oct 30. Oncogene. 2018. PMID: 29084211 Free PMC article.
-
Lauric acid promotes neuronal maturation mediated by astrocytes in primary cortical cultures.Heliyon. 2020 May 11;6(5):e03892. doi: 10.1016/j.heliyon.2020.e03892. eCollection 2020 May. Heliyon. 2020. PMID: 32420479 Free PMC article.
References
-
- Bagri A., Gurney T., He X., Zou Y. R., Littman D. R., Tessier-Lavigne M., et al. (2002). The chemokine SDF1 regulates migration of dentate granule cells. Development 129, 4249–4260 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

