Imaging the microanatomy of astrocyte-T-cell interactions in immune-mediated inflammation
- PMID: 23641198
- PMCID: PMC3639405
- DOI: 10.3389/fncel.2013.00058
Imaging the microanatomy of astrocyte-T-cell interactions in immune-mediated inflammation
Abstract
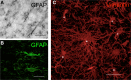
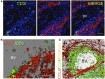
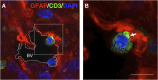
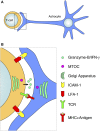
The role of astrocytes in the immune-mediated inflammatory response in the brain is more prominent than previously thought. Astrocytes become reactive in response to neuro-inflammatory stimuli through multiple pathways, contributing significantly to the machinery that modifies the parenchymal environment. In particular, astrocytic signaling induces the establishment of critical relationships with infiltrating blood cells, such as lymphocytes, which is a fundamental process for an effective immune response. The interaction between astrocytes and T-cells involves complex modifications to both cell types, which undergo micro-anatomical changes and the redistribution of their binding and secretory domains. These modifications are critical for different immunological responses, such as for the effectiveness of the T-cell response, for the specific infiltration of these cells and their homing in the brain parenchyma, and for their correct apposition with antigen-presenting cells (APCs) to form immunological synapses (ISs). In this article, we review the current knowledge of the interactions between T-cells and astrocytes in the context of immune-mediated inflammation in the brain, based on the micro-anatomical imaging of these appositions by high-resolution confocal microscopy and three-dimensional rendering. The study of these dynamic interactions using detailed technical approaches contributes to understanding the function of astrocytes in inflammatory responses and paves the way for new therapeutic strategies.
Keywords: T-cell; astrocyte; chemokines; cytokines; glioma; immune response; immunological synapse; infiltration.
Figures



Similar articles
-
T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury.PLoS One. 2008 Aug 20;3(8):e2977. doi: 10.1371/journal.pone.0002977. PLoS One. 2008. PMID: 18714338 Free PMC article.
-
p38α (MAPK14) critically regulates the immunological response and the production of specific cytokines and chemokines in astrocytes.Sci Rep. 2014 Dec 12;4:7405. doi: 10.1038/srep07405. Sci Rep. 2014. PMID: 25502009 Free PMC article.
-
Studying the T Cell-Astrocyte Immune Synapse.Methods Mol Biol. 2017;1584:517-531. doi: 10.1007/978-1-4939-6881-7_32. Methods Mol Biol. 2017. PMID: 28255723
-
Immune function of astrocytes.Glia. 2001 Nov;36(2):180-90. doi: 10.1002/glia.1107. Glia. 2001. PMID: 11596126 Review.
-
Astrocyte immune responses in epilepsy.Glia. 2012 Aug;60(8):1258-68. doi: 10.1002/glia.22312. Epub 2012 Feb 13. Glia. 2012. PMID: 22331574 Review.
Cited by
-
Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10.Eur J Immunol. 2015 Jan;45(1):180-91. doi: 10.1002/eji.201444823. Epub 2014 Nov 24. Eur J Immunol. 2015. PMID: 25329858 Free PMC article.
-
A Xenotransplant Model of Human Brain Tumors in Wild-Type Mice.iScience. 2020 Jan 24;23(1):100813. doi: 10.1016/j.isci.2019.100813. Epub 2019 Dec 30. iScience. 2020. PMID: 31931429 Free PMC article.
-
Bidirectional modulation between infiltrating CD3+ T-lymphocytes and astrocytes in the spinal cord drives the development of allodynia in monoarthritic rats.Sci Rep. 2018 Jan 8;8(1):51. doi: 10.1038/s41598-017-18357-z. Sci Rep. 2018. PMID: 29311654 Free PMC article.
-
Neuroimmunological Implications of AQP4 in Astrocytes.Int J Mol Sci. 2016 Aug 10;17(8):1306. doi: 10.3390/ijms17081306. Int J Mol Sci. 2016. PMID: 27517922 Free PMC article. Review.
-
CREB signals as PBMC-based biomarkers of cognitive dysfunction: A novel perspective of the brain-immune axis.Brain Behav Immun. 2019 May;78:9-20. doi: 10.1016/j.bbi.2019.01.004. Epub 2019 Jan 12. Brain Behav Immun. 2019. PMID: 30641141 Free PMC article. Review.
References
-
- Barcia C., Sanderson N. S., Barrett R. J., Wawrowsky K., Kroeger K. M., Puntel M., et al. (2008a). T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS ONE 3:e2977 10.1371/journal.pone.0002977 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

