Characterization and ex vivo Expansion of Human Placenta-Derived Natural Killer Cells for Cancer Immunotherapy
- PMID: 23641243
- PMCID: PMC3640206
- DOI: 10.3389/fimmu.2013.00101
Characterization and ex vivo Expansion of Human Placenta-Derived Natural Killer Cells for Cancer Immunotherapy
Abstract
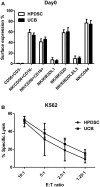
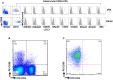
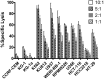
Recent clinical studies suggest that adoptive transfer of donor-derived natural killer (NK) cells may improve clinical outcome in hematological malignancies and some solid tumors by direct anti-tumor effects as well as by reduction of graft versus host disease (GVHD). NK cells have also been shown to enhance transplant engraftment during allogeneic hematopoietic stem cell transplantation (HSCT) for hematological malignancies. The limited ex vivo expansion potential of NK cells from peripheral blood (PB) or umbilical cord blood (UCB) has however restricted their therapeutic potential. Here we define methods to efficiently generate NK cells from donor-matched, full-term human placenta perfusate (termed Human Placenta-Derived Stem Cell, HPDSC) and UCB. Following isolation from cryopreserved donor-matched HPDSC and UCB units, CD56+CD3- placenta-derived NK cells, termed pNK cells, were expanded in culture for up to 3 weeks to yield an average of 1.2 billion cells per donor that were >80% CD56+CD3-, comparable to doses previously utilized in clinical applications. Ex vivo-expanded pNK cells exhibited a marked increase in anti-tumor cytolytic activity coinciding with the significantly increased expression of NKG2D, NKp46, and NKp44 (p < 0.001, p < 0.001, and p < 0.05, respectively). Strong cytolytic activity was observed against a wide range of tumor cell lines in vitro. pNK cells display a distinct microRNA (miRNA) expression profile, immunophenotype, and greater anti-tumor capacity in vitro compared to PB NK cells used in recent clinical trials. With further development, pNK may represent a novel and effective cellular immunotherapy for patients with high clinical needs and few other therapeutic options.
Keywords: anti-tumor cytolytic activity; cellular immunotherapy; ex vivo expansion; miRNA; placental-derived natural killer cells.
Figures


Similar articles
-
Ex vivo expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells through different receptor-ligand interactions.J Exp Clin Cancer Res. 2021 Oct 23;40(1):333. doi: 10.1186/s13046-021-02089-0. J Exp Clin Cancer Res. 2021. PMID: 34686187 Free PMC article.
-
A novel method to expand large numbers of CD56(+) natural killer cells from a minute fraction of selectively accessed cryopreserved cord blood for immunotherapy after transplantation.Cytotherapy. 2015 Nov;17(11):1582-93. doi: 10.1016/j.jcyt.2015.07.020. Cytotherapy. 2015. PMID: 26432560 Free PMC article.
-
Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial.Biol Blood Marrow Transplant. 2016 Jul;22(7):1290-1298. doi: 10.1016/j.bbmt.2016.04.009. Epub 2016 Apr 16. Biol Blood Marrow Transplant. 2016. PMID: 27090958 Free PMC article. Clinical Trial.
-
[Umbilical cord blood as a source of stem cells].Acta Med Croatica. 2006 Jun;60(3):215-25. Acta Med Croatica. 2006. PMID: 16933834 Review. Croatian.
-
Cytotoxic function of umbilical cord blood natural killer cells: relevance to adoptive immunotherapy.Pediatr Hematol Oncol. 2011 Nov;28(8):640-6. doi: 10.3109/08880018.2011.613092. Epub 2011 Oct 4. Pediatr Hematol Oncol. 2011. PMID: 21970456 Review.
Cited by
-
Reprogramming natural killer cells for cancer therapy.Mol Ther. 2024 Sep 4;32(9):2835-2855. doi: 10.1016/j.ymthe.2024.01.027. Epub 2024 Jan 24. Mol Ther. 2024. PMID: 38273655 Review.
-
Human placental hematopoietic stem cell derived natural killer cells (CYNK-001) mediate protection against influenza a viral infection.Hum Vaccin Immunother. 2022 Nov 30;18(5):2055945. doi: 10.1080/21645515.2022.2055945. Epub 2022 Apr 11. Hum Vaccin Immunother. 2022. PMID: 35404743 Free PMC article.
-
Decoding the multidimensional signatures of resident and expanded natural killer cells generated from perinatal blood.Am J Cancer Res. 2022 May 15;12(5):2132-2145. eCollection 2022. Am J Cancer Res. 2022. PMID: 35693070 Free PMC article.
-
NK cell-based cancer immunotherapy: from basic biology to clinical development.J Hematol Oncol. 2021 Jan 6;14(1):7. doi: 10.1186/s13045-020-01014-w. J Hematol Oncol. 2021. PMID: 33407739 Free PMC article. Review.
-
Senescence, NK cells, and cancer: navigating the crossroads of aging and disease.Front Immunol. 2025 Apr 4;16:1565278. doi: 10.3389/fimmu.2025.1565278. eCollection 2025. Front Immunol. 2025. PMID: 40255394 Free PMC article. Review.
References
-
- Chiffoleau E., Kobayashi T., Walsh M. C., King C. G., Walsh P. T., Hancock W. W., et al. (2003). TNF receptor-associated factor 6 deficiency during hemopoiesis induces Th2-polarized inflammatory disease. J. Immunol. 171, 5751–5759 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

