Design and Evaluation of Novel Antimicrobial and Anticancer Agents Among Tetrazolo[1,5-c]quinazoline-5-thione S-Derivatives
- PMID: 23641327
- PMCID: PMC3617672
- DOI: 10.3797/scipharm.1208-13
Design and Evaluation of Novel Antimicrobial and Anticancer Agents Among Tetrazolo[1,5-c]quinazoline-5-thione S-Derivatives
Abstract
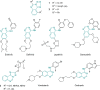
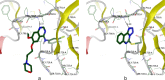
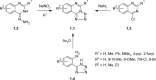
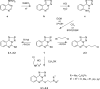
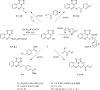
The novel heterocyclization of 5-(2-aminophenyl)-1H-tetrazole with potassium ethylxanthogenate or carbon disulfide was proposed. The potassium salt of the tetrazolo[1,5-c]quinazoline-5-thione was subsequently modified by alkylation with proper halogen derivatives to (tetrazolo[1,5-c]quinazolin-5-ylthio)alkyls, N,N-dialkylethylamines, 1-aryl-2-ethanones, 1-(alkyl)aryl-2-ethanols, carboxylic acids, and esters. The structures of all newly synthesized compounds were confirmed by FT-IR, UV-vis, LC-MS, (1)H, (13)C NMR, and elemental analysis data. The substances were screened for antibacterial and antifungal activities (100 μg) against Escherichia coli, Staphylococcus aureus, Enterobacter aerogenes, Entrococcus faecalis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida albicans. Preliminary bioluminescence inhibition tests against Photobacterium leiognathi Sh1 showed that substances 5.2-5.4, 6.1, 7.1 with ethanone or carboxylic acid substituents showed toxicity against bacteria cells. The substances chosen by the US National Cancer Institute (NCI) were screened for their ability to inhibit 60 different human tumor cell lines, where 2-(tetrazolo[1,5-c]quinazolin-5-ylthio)-1-(4-tolyl)ethanone (5.2), 3-(tetrazolo[1,5-c]quinazolin-5-ylthio)propanoic and related 3-metyl-butanoic acids (6.2, 6.3), and ethyl tetrazolo[1,5-c]quinazolin-5-ylthio)acetate (7.2) showed lethal antitumor activity (1.0 μM) against the acute lymphoblastic leukemia cell line (CCRF-CEM), and substances 5.2 and 6.3 exhibited moderate anticancer properties inhibiting growth of the leukemia MOLT-4 and HL06-(TB) cell lines. The moderate antitumor activity was demonstrated in 1-(2,5-dimethoxyphenyl)-2-(tetrazolo[1,5-c]quinazolin-5-ylthio)ethanone (5.4) against the CNS cancer cell line SNB-75. Comparing the docking mode of the Gefitinib and synthesised substances on the ATP binding site of EGFR, it could be assumed that these compounds might act in the same way. The results of the investigation could be considered as a useful base for future development of potent antimicrobials and antitumor agents among tetrazolo[1,5-c]quinazoline-5-thione S-derivatives.
Keywords: 5-R-tetrazolo[1,5-c]quinazoline-5-thiones; Antibacterial; Anticancer; Antifungal; Bioluminescence.
Figures
References
-
- Ostrovskii VA, Koldobskii GI, Trifonov RE. 6.07: Tetrazoles. Compr Heterocycl Chem III. 2008;6:257–423. http://dx.doi.org/10.1016/B978-008044992-0.00517-4. - DOI
-
- Moderhack D. Ring Transformations in Tetrazole Chemistry. J Prakt Chem. 1998;340:687–709. http://dx.doi.org/10.1002/prac.19983400802. - DOI
-
- Singh H, Chawla AS, Kapoor VK, Paul D, Malhotra RK. Medicinal Chemistry of Tetrazoles. Ellis GP, West GB, editors. Progr Med Chem. 1980;17:151–183. http://dx.doi.org/10.1016/S0079-6468(08)70159-0. - DOI - PubMed
-
- Myznikov LV, Hrabalek A, Koldobskii GI. Drugs in the tetrazole series. (Review) Chem Heterocycl Comp. 2007;43:1–9. http://dx.doi.org/10.1007/s10593-007-0001-5. - DOI
-
- Sherif AFR, Hayam MAA, Heba AAR, Abd EFH, Abd EF. Azole antimicrobial pharmacophore-based tetrazoles: Synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg Med Chem. 2009;17:2410–2422. http://dx.doi.org/10.1016/j.bmc.2009.02.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
