Stochastic Hypothesis of Transition from Inborn Neutropenia to AML: Interactions of Cell Population Dynamics and Population Genetics
- PMID: 23641360
- PMCID: PMC3638131
- DOI: 10.3389/fonc.2013.00089
Stochastic Hypothesis of Transition from Inborn Neutropenia to AML: Interactions of Cell Population Dynamics and Population Genetics
Abstract
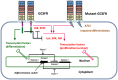
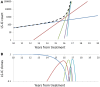
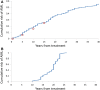
We present a stochastic model of driver mutations in the transition from severe congenital neutropenia to myelodysplastic syndrome to acute myeloid leukemia (AML). The model has the form of a multitype branching process. We derive equations for the distributions of the times to consecutive driver mutations and set up simulations involving a range of hypotheses regarding acceleration of the mutation rates in successive mutant clones. Our model reproduces the clinical distribution of times at diagnosis of secondary AML. Surprisingly, within the framework of our assumptions, stochasticity of the mutation process is incapable of explaining the spread of times at diagnosis of AML in this case; it is necessary to additionally assume a wide spread of proliferative parameters among disease cases. This finding is unexpected but generally consistent with the wide heterogeneity of characteristics of human cancers.
Keywords: acute myeloid leukemia; branching process; clonal evolution; driver mutations; myelodysplastic syndrome; severe congenital neutropenia.
Figures

Similar articles
-
Application of the Moran Model in Estimating Selection Coefficient of Mutated CSF3R Clones in the Evolution of Severe Congenital Neutropenia to Myeloid Neoplasia.Front Physiol. 2020 Sep 17;11:806. doi: 10.3389/fphys.2020.00806. eCollection 2020. Front Physiol. 2020. PMID: 33041834 Free PMC article.
-
Game of clones: the genomic evolution of severe congenital neutropenia.Hematology Am Soc Hematol Educ Program. 2015;2015:1-7. doi: 10.1182/asheducation-2015.1.1. Hematology Am Soc Hematol Educ Program. 2015. PMID: 26637693
-
Sequential gain of mutations in severe congenital neutropenia progressing to acute myeloid leukemia.Blood. 2012 May 31;119(22):5071-7. doi: 10.1182/blood-2012-01-406116. Epub 2012 Feb 27. Blood. 2012. PMID: 22371884 Clinical Trial.
-
Risk of myelodysplastic syndrome and acute myeloid leukemia in congenital neutropenias.Semin Hematol. 2002 Apr;39(2):128-33. doi: 10.1053/shem.2002.31912. Semin Hematol. 2002. PMID: 11957196 Review.
-
[Gene Mutation and Acute Leukemia Transformation of Severe Congenital Neutropenia- Review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017 Oct;25(5):1580-1584. doi: 10.7534/j.issn.1009-2137.2017.05.053. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017. PMID: 29070147 Review. Chinese.
Cited by
-
Application of the Moran Model in Estimating Selection Coefficient of Mutated CSF3R Clones in the Evolution of Severe Congenital Neutropenia to Myeloid Neoplasia.Front Physiol. 2020 Sep 17;11:806. doi: 10.3389/fphys.2020.00806. eCollection 2020. Front Physiol. 2020. PMID: 33041834 Free PMC article.
-
Mutation, drift and selection in single-driver hematologic malignancy: Example of secondary myelodysplastic syndrome following treatment of inherited neutropenia.PLoS Comput Biol. 2019 Jan 7;15(1):e1006664. doi: 10.1371/journal.pcbi.1006664. eCollection 2019 Jan. PLoS Comput Biol. 2019. PMID: 30615612 Free PMC article.
-
Simulating cancer: computational models in oncology.Front Oncol. 2013 Sep 13;3:233. doi: 10.3389/fonc.2013.00233. eCollection 2013. Front Oncol. 2013. PMID: 24062986 Free PMC article. No abstract available.
-
Phenotypic equilibrium as probabilistic convergence in multi-phenotype cell population dynamics.PLoS One. 2017 Feb 9;12(2):e0170916. doi: 10.1371/journal.pone.0170916. eCollection 2017. PLoS One. 2017. PMID: 28182672 Free PMC article.
-
Tumor evolution: Linear, branching, neutral or punctuated?Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):151-161. doi: 10.1016/j.bbcan.2017.01.003. Epub 2017 Jan 19. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28110020 Free PMC article. Review.
References
-
- Bender J. G., Unverzagt K., Walker D. E., Lee W., Smith S., Williams S., et al. (1994). Phenotypic analysis and characterization of CD34+ cells from normal human bone marrow, cord blood, peripheral blood, and mobilized peripheral blood from patients undergoing autologous stem cell transplantation. Clin. Immunol. Immunopathol. 70, 10–1810.1006/clin.1994.1003 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

