Engineering cyanobacteria to improve photosynthetic production of alka(e)nes
- PMID: 23641684
- PMCID: PMC3679977
- DOI: 10.1186/1754-6834-6-69
Engineering cyanobacteria to improve photosynthetic production of alka(e)nes
Abstract
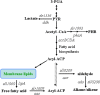
Background: Cyanobacteria can utilize solar energy and convert carbon dioxide into biofuel molecules in one single biological system. Synechocystis sp. PCC 6803 is a model cyanobacterium for basic and applied research. Alkanes are the major constituents of gasoline, diesel and jet fuels. A two-step alkane biosynthetic pathway was identified in cyanobacteria recently. It opens a door to achieve photosynthetic production of alka(e)nes with high efficiency by genetically engineering cyanobacteria.
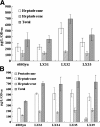
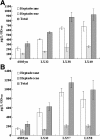
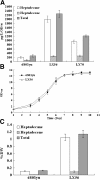
Results: A series of Synechocystis sp. PCC6803 mutant strains have been constructed and confirmed. Overexpression of both acyl-acyl carrier protein reductase and aldehyde-deformylating oxygenase from several cyanobacteria strains led to a doubled alka(e)ne production. Redirecting the carbon flux to acyl- ACP can provide larger precursor pool for further conversion to alka(e)nes. In combination with the overexpression of alkane biosynthetic genes, alka(e)ne production was significantly improved in these engineered strains. Alka(e)ne content in a Synechocystis mutant harboring alkane biosynthetic genes over-expressed in both slr0168 and slr1556 gene loci (LX56) was 1.3% of cell dry weight, which was enhanced by 8.3 times compared with wildtype strain (0.14% of cell dry weight) cultivated in shake flasks. Both LX56 mutant and the wildtype strain were cultivated in column photo-bioreactors, and the alka(e)ne production in LX56 mutant was 26 mg/L (1.1% of cell dry weight), which was enhanced by 8 times compared with wildtype strain (0.13% of cell dry weight).
Conclusions: The extent of alka(e)ne production could correlate positively with the expression level of alkane biosynthetic genes. Redirecting the carbon flux to acyl-ACP and overexpressing alkane biosynthetic genes simultaneously can enhance alka(e)ne production in cyanobacteria effectively.
Figures
Similar articles
-
High Light Induced Alka(e)ne Biodegradation for Lipid and Redox Homeostasis in Cyanobacteria.Front Microbiol. 2020 Jul 17;11:1659. doi: 10.3389/fmicb.2020.01659. eCollection 2020. Front Microbiol. 2020. PMID: 32765469 Free PMC article.
-
Engineering acyl-ACP reductase with fusion tags enhances alka(e)ne synthesis in Escherichia coli.Enzyme Microb Technol. 2023 Aug;168:110262. doi: 10.1016/j.enzmictec.2023.110262. Epub 2023 May 19. Enzyme Microb Technol. 2023. PMID: 37224590
-
Improved production of fatty alcohols in cyanobacteria by metabolic engineering.Biotechnol Biofuels. 2014 Jun 18;7:94. doi: 10.1186/1754-6834-7-94. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25024742 Free PMC article.
-
Microbial Synthesis of Alka(e)nes.Front Bioeng Biotechnol. 2013 Oct 16;1:10. doi: 10.3389/fbioe.2013.00010. eCollection 2013. Front Bioeng Biotechnol. 2013. PMID: 25023719 Free PMC article. Review.
-
Recent advances in the improvement of cyanobacterial enzymes for bioalkane production.Microb Cell Fact. 2022 Dec 12;21(1):256. doi: 10.1186/s12934-022-01981-4. Microb Cell Fact. 2022. PMID: 36503511 Free PMC article. Review.
Cited by
-
Biosynthesis of hydrocarbons and volatile organic compounds by fungi: bioengineering potential.Appl Microbiol Biotechnol. 2015 Jun;99(12):4943-51. doi: 10.1007/s00253-015-6641-y. Epub 2015 May 10. Appl Microbiol Biotechnol. 2015. PMID: 25957494 Free PMC article. Review.
-
Exploring native genetic elements as plug-in tools for synthetic biology in the cyanobacterium Synechocystis sp. PCC 6803.Microb Cell Fact. 2018 Mar 26;17(1):48. doi: 10.1186/s12934-018-0897-8. Microb Cell Fact. 2018. PMID: 29580240 Free PMC article.
-
Photosynthetic Conversion of Carbon Dioxide to Oleochemicals by Cyanobacteria: Recent Advances and Future Perspectives.Front Microbiol. 2020 Apr 17;11:634. doi: 10.3389/fmicb.2020.00634. eCollection 2020. Front Microbiol. 2020. PMID: 32362881 Free PMC article. Review.
-
Improved Alkane Production in Nitrogen-Fixing and Halotolerant Cyanobacteria via Abiotic Stresses and Genetic Manipulation of Alkane Synthetic Genes.Curr Microbiol. 2015 Jul;71(1):115-20. doi: 10.1007/s00284-015-0833-7. Epub 2015 May 14. Curr Microbiol. 2015. PMID: 25971893
-
Identification of two genes required for heptadecane production in a N2-fixing cyanobacterium Anabaena sp. strain PCC 7120.AMB Express. 2018 Oct 13;8(1):167. doi: 10.1186/s13568-018-0700-6. AMB Express. 2018. PMID: 30317393 Free PMC article.
References
-
- Dexter J, Fu PC. Metabolic engineering of cyanobacteria for ethanol production. Energ Environ Sci. 2009;2:857–864. doi: 10.1039/b811937f. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

