S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington's disease
- PMID: 23641925
- PMCID: PMC3785802
- DOI: 10.1089/ars.2012.4928
S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington's disease
Abstract
Aims: Dynamin-related protein1 (Drp1) is a large GTPase that mediates mitochondrial fission. We recently reported in Alzheimer's disease (AD) that S-nitrosylation of Drp1 (forming S-nitroso [SNO]-Drp1) results in GTPase hyperactivity and mitochondrial fragmentation, thus impairing bioenergetics and inducing synaptic damage and neuronal loss. Here, since aberrant mitochondrial dynamics are also key features of Huntington's disease (HD), we investigated whether formation of SNO-Drp1 contributes to the pathogenesis of HD in cell-based and animal models.
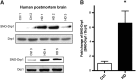
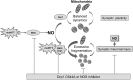
Results: We found that expression of mutant huntingtin (mutHTT) protein in primary cultured neurons triggers significant production of nitric oxide (NO). Consistent with this result, increased levels of SNO-Drp1 were found in the striatum of a transgenic mouse model of HD as well as in human postmortem brains from HD patients. Using specific fluorescence markers, we found that formation of SNO-Drp1 induced excessive mitochondrial fragmentation followed by loss of dendritic spines, signifying synaptic damage. These neurotoxic events were significantly abrogated after transfection with non-nitrosylatable mutant Drp1(C644A), or by the blocking of NO production using an nitric oxide synthase inhibitor. These findings suggest that SNO-Drp1 is a key mediator of mutHTT toxicity, and, thus, may represent a novel drug target for HD.
Innovation and conclusion: Our findings indicate that aberrant S-nitrosylation of Drp1 is a prominent pathological feature of neurodegenerative diseases such as AD and HD. Moreover, the SNO-Drp1 signaling pathway links mutHTT neurotoxicity to a malfunction in mitochondrial dynamics, resulting in neuronal synaptic damage in HD.
Figures






Similar articles
-
Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease.Hum Mol Genet. 2012 Jan 15;21(2):406-20. doi: 10.1093/hmg/ddr475. Epub 2011 Oct 13. Hum Mol Genet. 2012. PMID: 21997870 Free PMC article.
-
Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity.Nat Med. 2011 Mar;17(3):377-82. doi: 10.1038/nm.2313. Epub 2011 Feb 20. Nat Med. 2011. PMID: 21336284 Free PMC article.
-
Drp1 phosphorylation by MAPK1 causes mitochondrial dysfunction in cell culture model of Huntington's disease.Biochem Biophys Res Commun. 2018 Feb 5;496(2):706-711. doi: 10.1016/j.bbrc.2018.01.114. Epub 2018 Jan 31. Biochem Biophys Res Commun. 2018. PMID: 29397067 Free PMC article.
-
S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration.Mitochondrion. 2010 Aug;10(5):573-8. doi: 10.1016/j.mito.2010.04.007. Epub 2010 May 4. Mitochondrion. 2010. PMID: 20447471 Free PMC article. Review.
-
Increased mitochondrial fission and neuronal dysfunction in Huntington's disease: implications for molecular inhibitors of excessive mitochondrial fission.Drug Discov Today. 2014 Jul;19(7):951-5. doi: 10.1016/j.drudis.2014.03.020. Epub 2014 Mar 28. Drug Discov Today. 2014. PMID: 24681059 Free PMC article. Review.
Cited by
-
Mitochondrial protein dysfunction in pathogenesis of neurological diseases.Front Mol Neurosci. 2022 Sep 7;15:974480. doi: 10.3389/fnmol.2022.974480. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157077 Free PMC article. Review.
-
Oxidative Cysteine Post Translational Modifications Drive the Redox Code Underlying Neurodegeneration and Amyotrophic Lateral Sclerosis.Antioxidants (Basel). 2024 Jul 23;13(8):883. doi: 10.3390/antiox13080883. Antioxidants (Basel). 2024. PMID: 39199129 Free PMC article. Review.
-
S-nitrosylation of E3 ubiquitin-protein ligase RNF213 alters non-canonical Wnt/Ca+2 signaling in the P301S mouse model of tauopathy.Transl Psychiatry. 2019 Jan 29;9(1):44. doi: 10.1038/s41398-019-0388-7. Transl Psychiatry. 2019. PMID: 30696811 Free PMC article.
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
How Do Post-Translational Modifications Influence the Pathomechanistic Landscape of Huntington's Disease? A Comprehensive Review.Int J Mol Sci. 2020 Jun 16;21(12):4282. doi: 10.3390/ijms21124282. Int J Mol Sci. 2020. PMID: 32560122 Free PMC article. Review.
References
-
- Arrasate M. Mitra S. Schweitzer ES. Segal MR. Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. - PubMed
-
- Barsoum MJ. Yuan H. Gerencser AA. Liot G. Kushnareva Y. Graber S. Kovacs I. Lee WD. Waggoner J. Cui J. White AD. Bossy B. Martinou JC. Youle RJ. Lipton SA. Ellisman MH. Perkins GA. Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. - PMC - PubMed
-
- Bossy-Wetzel E. Barsoum MJ. Godzik A. Schwarzenbacher R. Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

