Time course changes of anti- and pro-apoptotic proteins in apigenin-induced genotoxicity
- PMID: 23642018
- PMCID: PMC3660279
- DOI: 10.1186/1749-8546-8-9
Time course changes of anti- and pro-apoptotic proteins in apigenin-induced genotoxicity
Abstract
Background: Apigenin (4',5,7-trihydroxyflavone, AP), an active component of many medicinal Chinese herbs, exhibits anticancer properties in vitro and in vivo. This study aims to investigate the genotoxic, cytostatic, and cytotoxic effects of AP and time course changes in the levels of anti- and pro-apoptotic proteins involved in the DNA damage response in HepG2 cells.
Methods: The genotoxic potential of AP was determined by sister chromatid exchanges (SCEs) and chromosomal aberrations (CAs) analysis. The levels of cytostaticity and cytotoxicity were evaluated by the proliferation rate and mitotic indices, respectively. MTT was used to study cytotoxicity, while the induction of apoptosis and the expression of apoptosis-related proteins were determined by ELISA.
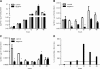
Results: At concentrations greater than 10 μM, AP decreased cell survival in a dose- (48 h: 10 vs. 20 μΜ, P < 0.001 and 20 vs. 50 μΜ, P = 0.005; 72 h: 10 vs. 20 μΜ, P < 0.001 and 20 vs. 50 μΜ, P = 0.001) and time-dependent manner (20 μΜ: 24 vs. 48 h, P < 0.001 and 48 vs. 72 h, P = 0.003; 50 μΜ: 24 vs. 48 h, P < 0.001 and 48 vs. 72 h, P < 0.001; 100 μΜ: 24 vs. 48 h, P < 0.001 and 48 vs. 72 h, P < 0.001). SCEs rates, cell proliferation, and mitotic divisions were also affected in a dose-dependent manner (P < 0.001). There was no change in the frequency of aberrant cells (1 μΜ ΑP: P = 0.554; 10 μM AP: P = 0.337; 20 μΜ AP: P = 0.239). Bcl-2 levels were reduced 3 h after AP administration (P = 0.003) and remained reduced throughout the 48 h observation period (6 h, P = 0.044; 12 h, P = 0.001; 24 h, P = 0.042; 48 h, P = 0.012). Bax and soluble Fas exhibited a transient upregulation 24 h after AP treatment. The Bax/Bcl-2 ratio was also increased at 12 h and remained increased throughout the 48 h observation period.
Conclusion: AP exhibited dose-dependent genotoxic potential in HepG2 cells. The protein levels of sFas, Bcl-2, and Bax were affected by AP to promote cell survival and cell death, respectively.
Figures
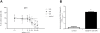
Similar articles
-
Assessment of cytotoxic and genotoxic effects of enniatin-A in vitro.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018 Aug;35(8):1633-1644. doi: 10.1080/19440049.2018.1486513. Epub 2018 Jul 16. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018. PMID: 29889654
-
Evaluation of beauvericin genotoxicity with the chromosomal aberrations, sister-chromatid exchanges and micronucleus assays.Ecotoxicol Environ Saf. 2010 Oct;73(7):1553-7. doi: 10.1016/j.ecoenv.2010.07.036. Epub 2010 Aug 13. Ecotoxicol Environ Saf. 2010. PMID: 20708264
-
Genotoxicity induced in vitro by water-soluble indoor PM2.5 fractions in relation to heavy metal concentrations.Environ Monit Assess. 2021 Jan 23;193(2):82. doi: 10.1007/s10661-021-08884-8. Environ Monit Assess. 2021. PMID: 33486539
-
Antigenotoxic effect of apigenin against mitomycin C induced genotoxic damage in mice bone marrow cells.Food Chem Toxicol. 2009 Mar;47(3):536-9. doi: 10.1016/j.fct.2008.12.006. Epub 2008 Dec 16. Food Chem Toxicol. 2009. PMID: 19121640
-
Genotoxicity of cisplatin and carboplatin in cultured human lymphocytes: a comparative study.Interdiscip Toxicol. 2019 Oct;12(2):93-97. doi: 10.2478/intox-2019-0011. Epub 2020 Feb 20. Interdiscip Toxicol. 2019. PMID: 32206030 Free PMC article.
Cited by
-
Critical roles of Rad54 in tolerance to apigenin-induced Top1-mediated DNA damage.Exp Ther Med. 2021 May;21(5):505. doi: 10.3892/etm.2021.9936. Epub 2021 Mar 18. Exp Ther Med. 2021. PMID: 33791014 Free PMC article.
-
Alpha-bisabolol, not a matter for cancer therapy. Commentary: "Research on the immunosuppressive activity of ingredients contained in sunscreens".Front Pharmacol. 2015 May 11;6:96. doi: 10.3389/fphar.2015.00096. eCollection 2015. Front Pharmacol. 2015. PMID: 26029105 Free PMC article. No abstract available.
References
-
- Kim DI, Lee TK, Lim IS, Kim H, Lee YC, Kim CH. Regulation of IGF-I production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: isolation of flavonoids of apigenin and luteolin as acting compounds. Toxicol Appl Pharmacol. 2005;205:213–224. doi: 10.1016/j.taap.2004.10.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

