Microglial activation decreases retention of the protease inhibitor saquinavir: implications for HIV treatment
- PMID: 23642074
- PMCID: PMC3651327
- DOI: 10.1186/1742-2094-10-58
Microglial activation decreases retention of the protease inhibitor saquinavir: implications for HIV treatment
Abstract
Background: Active HIV infection within the central nervous system (CNS) is confined primarily to microglia. The glial cell compartment acts as a viral reservoir behind the blood-brain barrier. It provides an additional roadblock to effective pharmacological treatment via expression of multiple drug efflux transporters, including P-glycoprotein. HIV/AIDS patients frequently suffer bacterial and viral co-infections, leading to deregulation of glial cell function and release of pro-inflammatory mediators including cytokines, chemokines, and nitric oxide.
Methods: To better define the role of inflammation in decreased HIV drug accumulation into CNS targets, accumulation of the antiretroviral saquinavir was examined in purified cultures of rodent microglia exposed to the prototypical inflammatory mediator lipopolysaccharide (LPS).
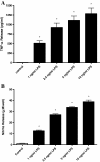
Results: [(3)H]-Saquinavir accumulation by microglia was rapid, and was increased up to two-fold in the presence of the specific P-glycoprotein inhibitor, PSC833. After six or 24 hours of exposure to 10 ng/ml LPS, saquinavir accumulation was decreased by up to 45%. LPS did not directly inhibit saquinavir transport, and did not affect P-glycoprotein protein expression. LPS exposure did not alter RNA and/or protein expression of other transporters including multidrug resistance-associated protein 1 and several solute carrier uptake transporters.
Conclusions: The decrease in saquinavir accumulation in microglia following treatment with LPS is likely multi-factorial, since drug accumulation was attenuated by inhibitors of NF-κβ and the MEK1/2 pathway in the microglia cell line HAPI, and in primary microglia cultures from toll-like receptor 4 deficient mice. These data provide new pharmacological insights into why microglia act as a difficult-to-treat viral sanctuary site.
Figures







References
-
- Highleyman L. HIV and the brain. BETA. 2009;21:16–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

