Role of cyclooxygenase-2 in exacerbation of allergen-induced airway remodeling by multiwalled carbon nanotubes
- PMID: 23642096
- PMCID: PMC3824045
- DOI: 10.1165/rcmb.2013-0019OC
Role of cyclooxygenase-2 in exacerbation of allergen-induced airway remodeling by multiwalled carbon nanotubes
Abstract
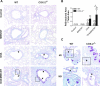
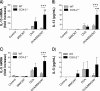
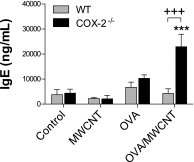
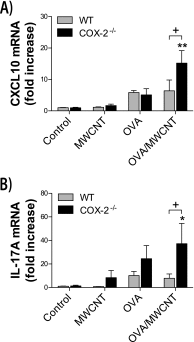
The emergence of nanotechnology has produced a multitude of engineered nanomaterials such as carbon nanotubes (CNTs), and concerns have been raised about their effects on human health, especially for susceptible populations such as individuals with asthma. Multiwalled CNTs (MWCNTs) have been shown to exacerbate ovalbumin (OVA)-induced airway remodeling in mice. Moreover, cyclooxygenase-2 (COX-2) has been described as a protective factor in asthma. We postulated that COX-2-deficient (COX-2(-/-)) mice would be susceptible to MWCNT-induced exacerbations of allergen-induced airway remodeling, including airway inflammation, fibrosis, and mucus-cell metaplasia (i.e., the formation of goblet cells). Wild-type (WT) or COX-2(-/-) mice were sensitized to OVA to induce allergic airway inflammation before a single dose of MWCNTs (4 mg/kg) delivered to the lungs by oropharyngeal aspiration. MWCNTs significantly increased OVA-induced lung inflammation and mucus-cell metaplasia in COX-2(-/-) mice compared with WT mice. However, airway fibrosis after exposure to allergen and MWCNTs was no different between WT and COX-2(-/-) mice. Concentrations of certain prostanoids (prostaglandin D2 and thromboxane B2) were enhanced by OVA or MWCNTs in COX-2(-/-) mice. No differences in COX-1 mRNA concentrations were evident between WT and COX-2(-/-) mice treated with OVA and MWCNTs. Interestingly, MWCNTs significantly enhanced allergen-induced cytokines involved in Th2 (IL-13 and IL-5), Th1 (CXCL10), and Th17 (IL-17A) inflammatory responses in COX-2(-/-) mice, but not in WT mice. We conclude that exacerbations of allergen-induced airway inflammation and mucus-cell metaplasia by MWCNTs are enhanced by deficiencies in COX-2, and are associated with the activation of a mixed Th1/Th2/Th17 immune response.
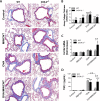
Figures


References
-
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report. J Allergy Clin Immunol. 2007;120:S94–S138. - PubMed
-
- Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225. - PubMed
-
- Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. - PubMed
-
- Gavett SH, Koren HS. The role of particulate matter in exacerbation of atopic asthma. Int Arch Allergy Immunol. 2001;124:109–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

