Optimization of transplastomic production of hemicellulases in tobacco: effects of expression cassette configuration and tobacco cultivar used as production platform on recombinant protein yields
- PMID: 23642171
- PMCID: PMC3655837
- DOI: 10.1186/1754-6834-6-65
Optimization of transplastomic production of hemicellulases in tobacco: effects of expression cassette configuration and tobacco cultivar used as production platform on recombinant protein yields
Abstract
Background: Chloroplast transformation in tobacco has been used extensively to produce recombinant proteins and enzymes. Chloroplast expression cassettes can be designed with different configurations of the cis-acting elements that govern foreign gene expression. With the aim to optimize production of recombinant hemicellulases in transplastomic tobacco, we developed a set of cassettes that incorporate elements known to facilitate protein expression in chloroplasts and examined expression and accumulation of a bacterial xylanase XynA. Biomass production is another important factor in achieving sustainable and high-volume production of cellulolytic enzymes. Therefore, we compared productivity of two tobacco cultivars - a low-alkaloid and a high-biomass - as transplastomic expression platforms.
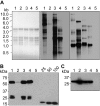
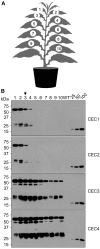
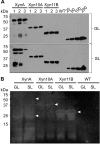
Results: Four different cassettes expressing XynA produced various mutant phenotypes of the transplastomic plants, affected their growth rate and resulted in different accumulation levels of the XynA enzyme. The most productive cassette was identified and used further to express XynA and two additional fungal xylanases, Xyn10A and Xyn11B, in a high-biomass tobacco cultivar. The high biomass cultivar allowed for a 60% increase in XynA production per plant. Accumulation of the fungal enzymes reached more than 10-fold higher levels than the bacterial enzyme, constituting up to 6% of the total soluble protein in the leaf tissue. Use of a well-characterized translational enhancer with the selected expression cassette revealed inconsistent effects on accumulation of the recombinant xylanases. Additionally, differences in the enzymatic activity of crude plant extracts measured in leaves of different age suggest presence of a specific xylanase inhibitor in the green leaf tissue.
Conclusion: Our results demonstrate the pivotal importance of the expression cassette design and appropriate tobacco cultivar for high-level transplastomic production of recombinant proteins.
Figures




Similar articles
-
High-level expression of thermostable cellulolytic enzymes in tobacco transplastomic plants and their use in hydrolysis of an industrially pretreated Arundo donax L. biomass.Biotechnol Biofuels. 2016 Jul 22;9:154. doi: 10.1186/s13068-016-0569-z. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 27453729 Free PMC article.
-
Expression of Acidothermus cellulolyticus E1 endo-beta-1,4-glucanase catalytic domain in transplastomic tobacco.Plant Biotechnol J. 2009 Aug;7(6):527-36. doi: 10.1111/j.1467-7652.2009.00421.x. Epub 2009 Jun 4. Plant Biotechnol J. 2009. PMID: 19500296
-
Production of leafy biomass using temporary immersion bioreactors: an alternative platform to express proteins in transplastomic plants with drastic phenotypes.Planta. 2013 Mar;237(3):903-8. doi: 10.1007/s00425-012-1829-1. Epub 2012 Dec 22. Planta. 2013. PMID: 23262582 Free PMC article.
-
Bioproduction of human enzymes in transgenic tobacco.Ann N Y Acad Sci. 1996 May 25;792:62-71. doi: 10.1111/j.1749-6632.1996.tb32492.x. Ann N Y Acad Sci. 1996. PMID: 8678421 Review.
-
Three Parts of the Plant Genome: On the Way to Success in the Production of Recombinant Proteins.Plants (Basel). 2022 Dec 21;12(1):38. doi: 10.3390/plants12010038. Plants (Basel). 2022. PMID: 36616166 Free PMC article. Review.
Cited by
-
Cloning and Expression of TNF Related Apoptosis Inducing Ligand in Nicotiana tabacum.Iran J Pharm Res. 2015 Winter;14(1):189-201. Iran J Pharm Res. 2015. PMID: 25561925 Free PMC article.
-
Plastid Transformation: New Challenges in the Circular Economy Era.Int J Mol Sci. 2022 Dec 3;23(23):15254. doi: 10.3390/ijms232315254. Int J Mol Sci. 2022. PMID: 36499577 Free PMC article. Review.
-
Co-expression with the Type 3 Secretion Chaperone CesT from Enterohemorrhagic E. coli Increases Accumulation of Recombinant Tir in Plant Chloroplasts.Front Plant Sci. 2017 Mar 6;8:283. doi: 10.3389/fpls.2017.00283. eCollection 2017. Front Plant Sci. 2017. PMID: 28321227 Free PMC article.
-
Plastid Transformation: How Does it Work? Can it Be Applied to Crops? What Can it Offer?Int J Mol Sci. 2020 Jul 9;21(14):4854. doi: 10.3390/ijms21144854. Int J Mol Sci. 2020. PMID: 32659946 Free PMC article. Review.
-
Chloroplast Engineering: Fundamental Insights and Its Application in Amelioration of Environmental Stress.Appl Biochem Biotechnol. 2023 Apr;195(4):2463-2482. doi: 10.1007/s12010-022-03930-8. Epub 2022 Apr 28. Appl Biochem Biotechnol. 2023. PMID: 35484466 Review.
References
-
- Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA. 2002;99:12246–12251. doi: 10.1073/pnas.182432999. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

