Metal atom lability in polynuclear complexes
- PMID: 23642178
- PMCID: PMC3678548
- DOI: 10.1021/ic302694y
Metal atom lability in polynuclear complexes
Abstract
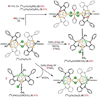
The asymmetric oxidation product [((Ph)L)Fe3(μ-Cl)]2 [(Ph)LH6 = MeC(CH2NHPh-o-NHPh)3], where each trinuclear core is comprised of an oxidized diiron unit [Fe2](5+) and an isolated trigonal pyramidal ferrous site, reacts with MCl2 salts to afford heptanuclear bridged structures of the type ((Ph)L)2Fe6M(μ-Cl)4(thf)2, where M = Fe or Co. Zero-field, (57)Fe Mössbauer analysis revealed the Co resides within the trinuclear core subunits, not at the octahedral, halide-bridged MCl4(thf)2 position indicating Co migration into the trinuclear subunits has occurred. Reaction of [((Ph)L)Fe3(μ-Cl)]2 with CoCl2 (2 or 5 equivalents) followed by precipitation via addition of acetonitrile afforded trinuclear products where one or two irons, respectively, can be substituted within the trinuclear core. Metal atom substitution was verified by (1)H NMR, (57)Fe Mossbauer, single crystal X-ray diffraction, X-ray fluorescence, and magnetometry analysis. Spectroscopic analysis revealed that the Co atom(s) substitute(s) into the oxidized dimetal unit ([M2](5+)), while the M(2+) site remains iron-substituted. Magnetic data acquired for the series are consistent with this analysis revealing the oxidized dimetal unit comprises a strongly coupled S = 1 unit ([FeCo](5+)) or S = 1/2 ([Co2](5+)) that is weakly antiferromagnetically coupled to the high spin (S = 2) ferrous site. The kinetic pathway for metal substitution was probed via reaction of [((Ph)L)Fe3(μ-Cl)]2 with isotopically enriched (57)FeCl2(thf)2, the results of which suggest rapid equilibration of (57)Fe into both the M(2+) site and oxidized diiron site, achieving a 1:1 mixture.
Figures
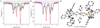
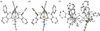
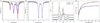
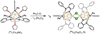

Similar articles
-
Site-isolated redox reactivity in a trinuclear iron complex.Inorg Chem. 2012 Oct 1;51(19):10274-8. doi: 10.1021/ic301241s. Epub 2012 Sep 18. Inorg Chem. 2012. PMID: 22988949 Free PMC article.
-
Structural, EPR, and Mössbauer characterization of (μ-alkoxo)(μ-carboxylato)diiron(II,III) model complexes for the active sites of mixed-valent diiron enzymes.Inorg Chem. 2012 Mar 5;51(5):2917-29. doi: 10.1021/ic2021726. Epub 2012 Feb 23. Inorg Chem. 2012. PMID: 22360600 Free PMC article.
-
Utilization of phosphinoamide ligands in homobimetallic Fe and Mn complexes: the effect of disparate coordination environments on metal-metal interactions and magnetic and redox properties.Inorg Chem. 2012 Aug 6;51(15):8225-40. doi: 10.1021/ic300776y. Epub 2012 Jul 17. Inorg Chem. 2012. PMID: 22804462
-
Catalytic C-H bond amination from high-spin iron imido complexes.J Am Chem Soc. 2011 Apr 6;133(13):4917-23. doi: 10.1021/ja110066j. Epub 2011 Mar 15. J Am Chem Soc. 2011. PMID: 21405138
-
New insights into the chemistry of di- and trimetallic iron dithiolene derivatives. Structural, Mössbauer, magnetic, electrochemical and theoretical studies.Dalton Trans. 2014 Sep 21;43(35):13187-95. doi: 10.1039/c4dt01462f. Dalton Trans. 2014. PMID: 25027173
Cited by
-
Nitric oxide activation facilitated by cooperative multimetallic electron transfer within an iron-functionalized polyoxovanadate-alkoxide cluster.Chem Sci. 2018 Jul 2;9(30):6379-6389. doi: 10.1039/c8sc00987b. eCollection 2018 Aug 14. Chem Sci. 2018. PMID: 30310566 Free PMC article.
-
Heterometallic bond activation enabled by unsymmetrical ligand scaffolds: bridging the opposites.Chem Sci. 2022 Oct 28;13(47):14008-14031. doi: 10.1039/d2sc04263k. eCollection 2022 Dec 7. Chem Sci. 2022. PMID: 36540828 Free PMC article. Review.
-
Synthesis of open-shell, bimetallic Mn/Fe trinuclear clusters.J Am Chem Soc. 2013 Sep 25;135(38):14448-58. doi: 10.1021/ja408003d. Epub 2013 Sep 11. J Am Chem Soc. 2013. PMID: 23984911 Free PMC article.
-
Cluster dynamics of heterometallic trinuclear clusters during ligand substitution, redox chemistry, and group transfer processes.Chem Sci. 2024 Feb 15;15(21):8242-8248. doi: 10.1039/d3sc03606e. eCollection 2024 May 29. Chem Sci. 2024. PMID: 38817579 Free PMC article.
-
Maximizing Electron Exchange in a [Fe3] Cluster.J Am Chem Soc. 2016 Feb 24;138(7):2235-43. doi: 10.1021/jacs.5b12181. Epub 2016 Feb 9. J Am Chem Soc. 2016. PMID: 26799500 Free PMC article.
References
-
-
Nitrogenase: Howard JB, Rees DC. Chem. Rev. 1996;96:2965. Burgess BK, Lowe DJ. Chem. Rev. 1996;96:2983–3012. Dos Santos PC, Igarashi RY, Lee H-I, Hoffman BM, Seefeldt LC, Dean DR. Acc. Chem. Res. 2005;38:208–214. Hoffman BM, Dean DR, Seefeldt LC. Acc. Chem. Res. 2009;42:609–619.
-
-
-
Photosystem II: Nugent J, editor. Biochim. Biophys. Acta. 2001;1503:1. Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831. Iwata S, Barber J. Curr. Opin. Struct. Biol. 2004;14:447.
-
-
-
Mn/Fe Ribonucleotide reductase: Jiang W, Yun D, Saleh L, Barr EW, Xing G, Hoffart LM, Maslak M-A, Krebs C, Bollinger JM., Jr Science. 2007;316:1188. Jiang W, Hoffart LM, Krebs C, Bollinger JM., Jr Biochemistry. 2007;46:8709.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

