Vitamin E trafficking in neurologic health and disease
- PMID: 23642196
- PMCID: PMC9719402
- DOI: 10.1146/annurev-nutr-071812-161252
Vitamin E trafficking in neurologic health and disease
Abstract
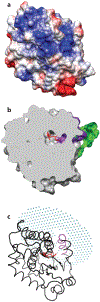
Vitamin E was identified almost a century ago as a botanical compound necessary for rodent reproduction. Decades of research since then established that of all members of the vitamin E family, α-tocopherol is selectively enriched in human tissues, and it is essential for human health. The major function of α-tocopherol is thought to be that of a lipid-soluble antioxidant that prevents oxidative damage to biological components. As such, α-tocopherol is necessary for numerous physiological processes such as permeability of lipid bilayers, cell adhesion, and gene expression. Inadequate levels of α-tocopherol interfere with cellular function and precipitate diseases, notably ones that affect the central nervous system. The extreme hydrophobicity of α-tocopherol poses a serious thermodynamic barrier for proper distribution of the vitamin to target tissues and cells. Although transport of the vitamin shares some steps with that of other lipids, selected tissues evolved dedicated transport mechanisms involving the α-tocopherol transfer protein (αTTP). The critical roles of this protein and its ligand are underscored by the debilitating pathologies that characterize human carriers of mutations in the TTPA gene.
Figures



References
-
- Atkinson J, Epand RF, Epand RM. 2008. Tocopherols and tocotrienols in membranes: a critical review. Free Radic. Biol. Med. 44:739–64 - PubMed
-
- Atkinson J, Harroun T, Wassall SR, Stillwell W, Katsaras J. 2010. The location and behavior of α-tocopherol in membranes. Mol. Nutr. Food Res. 54:641–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

