Malaria infection does not affect the sensitivity of peripheral receptor neurons in Anopheles stephensi
- PMID: 23642231
- PMCID: PMC3659000
- DOI: 10.1186/1756-3305-6-134
Malaria infection does not affect the sensitivity of peripheral receptor neurons in Anopheles stephensi
Abstract
Background: Mosquitoes transmit many important diseases including malaria, dengue and yellow fever. Disease transmission from one vertebrate host to another depends on repeated blood feedings by single mosquitoes. In order for the mosquito to acquire the blood that it needs to complete oogenesis, the insect must locate a suitable host. Olfactory cues (including carbon dioxide) released by the host and detected by the mosquito are the primary signals that vector insects use for host location. Previous studies have suggested that the physiological status - including bacterial, fungal, viral and Plasmodium infections - can modulate aspects of behavior in haematophagous insects.
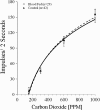
Methods: Standard electrophysiological techniques were used to record extracellular responses from the receptor neurons located in sensilla found on the maxillary palps of the insects. The recording microelectrode was inserted through the cuticle at the base of an individual sensillum and the extracellular electrical signals obtained from the three neurons within the sensillum were recorded. Stimulations consisted of 2 s pulses of the desired concentrations of CO(2) or dosages of 1-octen-3-ol.
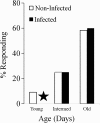
Results: Accordingly, we were interested in determining whether Plasmodium infection affects the sensitivity of those peripheral olfactory sensors that are involved in host-seeking in mosquitoes. Our studies indicate that infection of female Anopheles stephensi with Plasmodium berghei does not alter the response characteristics of the neurons innervating the maxillary palp sensilla that respond to the attractants carbon dioxide and 1-octen-3-ol. Although the response characteristics of the peripheral sensory neurons are not affected by infection status, we found that the age of the mosquito alone does affect the threshold of sensitivity of these neurons to carbon dioxide. The proportion of older insects (21-30 d post-emergence) that responds to 150 ppm carbon dioxide is higher than the proportion that responds among younger insects (1-10 d post-emergence).
Conclusions: Anopheles stephensi infected with Plasmodium berghei exhibit sensitivities to stimulation with carbon dioxide and 1-octen-3-ol similar to those of uninfected mosquitoes. However, the age of the infected or uninfected mosquito does affect the threshold of sensitivity of these neurons to carbon dioxide.
Figures




Similar articles
-
Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae.Curr Biol. 2007 Sep 18;17(18):1533-44. doi: 10.1016/j.cub.2007.07.062. Epub 2007 Aug 30. Curr Biol. 2007. PMID: 17764944 Free PMC article.
-
Reduction in host-finding behaviour in fungus-infected mosquitoes is correlated with reduction in olfactory receptor neuron responsiveness.Malar J. 2011 Aug 3;10:219. doi: 10.1186/1475-2875-10-219. Malar J. 2011. PMID: 21812944 Free PMC article.
-
Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections.Malar J. 2018 Oct 25;17(1):385. doi: 10.1186/s12936-018-2535-7. Malar J. 2018. PMID: 30359252 Free PMC article.
-
Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla.Ciba Found Symp. 1996;200:233-48; discussion 248-53, 281-4. doi: 10.1002/9780470514948.ch17. Ciba Found Symp. 1996. PMID: 8894301 Review.
-
Sensory bases of attractancy: morphology of mosquito olfactory sensilla-- a review.J Am Mosq Control Assoc. 1994 Jun;10(2 Pt 2):309-15. J Am Mosq Control Assoc. 1994. PMID: 8965084 Review.
Cited by
-
Transcriptome profiles of Anopheles gambiae harboring natural low-level Plasmodium infection reveal adaptive advantages for the mosquito.Sci Rep. 2021 Nov 19;11(1):22578. doi: 10.1038/s41598-021-01842-x. Sci Rep. 2021. PMID: 34799605 Free PMC article.
-
Olfaction in Anopheles mosquitoes.Chem Senses. 2021 Jan 1;46:bjab021. doi: 10.1093/chemse/bjab021. Chem Senses. 2021. PMID: 33885760 Free PMC article. Review.
References
-
- Takken W. The role of olfaction in host-seeking of mosquitoes: a review. Insect Sci Appl. 1991;12:287–295.
-
- Rudolfs W. Chemotropism of mosquitoes. N J Agric Experiment Stations Bull. 1922. pp. 5–23.
-
- Lambertsen CJ. In: Medical physiology. Mountcastle VB, editor. St Louis, MO: The C. V. Mosby Co; 1968. The atmosphere and gas exchanges with the lungs and blood; pp. 629–659.
-
- Grant AJ, Wigton BE, Aghajanian JG, O’Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A. 1995;177:389–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

