Proteomic analyses and identification of arginine methylated proteins differentially recognized by autosera from anti-Sm positive SLE patients
- PMID: 23642268
- PMCID: PMC3663782
- DOI: 10.1186/1423-0127-20-27
Proteomic analyses and identification of arginine methylated proteins differentially recognized by autosera from anti-Sm positive SLE patients
Abstract
Background: Antibodies against spliceosome Sm proteins (anti-Sm autoantibodies) are specific to the autoimmune disease systemic lupus erythematosus (SLE). Anti-Sm autosera have been reported to specifically recognize Sm D1 and D3 with symmetric di-methylarginines (sDMA). We investigated if anti-Sm sera from local SLE patients can differentially recognize Sm proteins or any other proteins due to their methylation states.
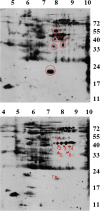
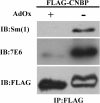
Results: We prepared HeLa cell proteins at normal or hypomethylation states (treated with an indirect methyltransferase inhibitor adenosine dialdehyde, AdOx). A few signals detected by the anti-Sm positive sera from typical SLE patients decreased consistently in the immunoblots of hypomethylated cell extracts. The differentially detected signals by one serum (Sm1) were pinpointed by two-dimensional electrophoresis and identified by mass spectrometry. Three identified proteins: splicing factor, proline- and glutamine-rich (SFPQ), heterogeneous nuclear ribonucleoprotein D-like (hnRNP DL) and cellular nucleic acid binding protein (CNBP) are known to contain methylarginines in their glycine and arginine rich (GAR) sequences. We showed that recombinant hnRNP DL and CNBP expressed in Escherichia coli can be detected by all anti-Sm positive sera we tested. As CNBP appeared to be differentially detected by the SLE sera in the pilot study, differential recognition of arginine methylated CNBP protein by the anti-Sm positive sera were further examined. Hypomethylated FLAG-CNBP protein immunopurified from AdOx-treated HeLa cells was less recognized by Sm1 compared to the CNBP protein expressed in untreated cells. Two of 20 other anti-Sm positive sera specifically differentiated the FLAG-CNBP protein expressed in HeLa cells due to the methylation. We also observed deferential recognition of methylated recombinant CNBP proteins expressed from E. coli by some of the autosera.
Conclusion: Our study showed that hnRNP DL and CNBP are novel antigens for SLE patients and the recognition of CNBP might be differentiated dependent on the level of arginine methylation.
Figures



Similar articles
-
Arginine methylation of the cellular nucleic acid binding protein does not affect its subcellular localization but impedes RNA binding.FEBS Lett. 2014 May 2;588(9):1542-8. doi: 10.1016/j.febslet.2014.03.052. Epub 2014 Apr 12. FEBS Lett. 2014. PMID: 24726729
-
Sequential autoantigenic determinants of the small nuclear ribonucleoprotein Sm D shared by human lupus autoantibodies and MRL lpr/lpr antibodies.Clin Exp Immunol. 1994 Dec;98(3):419-26. doi: 10.1111/j.1365-2249.1994.tb05507.x. Clin Exp Immunol. 1994. PMID: 7527740 Free PMC article.
-
Identification of autoantibodies to the I protein of the heterogeneous nuclear ribonucleoprotein complex in patients with systemic sclerosis.Arthritis Rheum. 1996 Oct;39(10):1669-76. doi: 10.1002/art.1780391009. Arthritis Rheum. 1996. PMID: 8843857
-
Autoantibodies to the A/B proteins of the heterogeneous nuclear ribonucleoprotein complex: novel tools for the diagnosis of rheumatic diseases.Int Arch Allergy Immunol. 1996 Dec;111(4):314-9. doi: 10.1159/000237386. Int Arch Allergy Immunol. 1996. PMID: 8957102 Review.
-
Clinical and immunological aspects of autoantibodies to RA33/hnRNP-A/B proteins--a link between RA, SLE and MCTD.Mol Biol Rep. 1996;23(3-4):167-71. doi: 10.1007/BF00351165. Mol Biol Rep. 1996. PMID: 9112225 Review.
Cited by
-
The Influence of Arginine Methylation in Immunity and Inflammation.J Inflamm Res. 2022 May 13;15:2939-2958. doi: 10.2147/JIR.S364190. eCollection 2022. J Inflamm Res. 2022. PMID: 35602664 Free PMC article. Review.
-
Oxidative Modifications in Tissue Pathology and Autoimmune Disease.Antioxid Redox Signal. 2018 Nov 10;29(14):1415-1431. doi: 10.1089/ars.2017.7382. Epub 2017 Dec 11. Antioxid Redox Signal. 2018. PMID: 29088923 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

