Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials
- PMID: 23642377
- PMCID: PMC3737287
- DOI: 10.1016/j.ophtha.2013.01.073
Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials
Abstract
Objective: To describe the effects of treatment for 1 year with ranibizumab or bevacizumab on macular morphology and the association of macular morphology with visual acuity (VA) in eyes with neovascular age-related macular degeneration (AMD).
Design: Prospective cohort study within a randomized clinical trial.
Participants: Participants in the Comparison of Age-related Macular Degeneration Treatments Trials.
Methods: Participants were assigned randomly to treatment with ranibizumab or bevacizumab on a monthly or as-needed schedule. Optical coherence tomography (OCT), fluorescein angiography (FA), color fundus photography (FP), and VA testing were performed periodically throughout 52 weeks. Masked readers graded images. General linear models were applied to evaluate effects of time and treatment on outcomes.
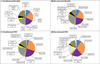
Main outcome measures: Fluid type and location and thickness by OCT, size, and lesion composition on FP, FA, and VA.
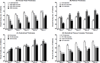
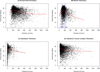
Results: Intraretinal fluid (IRF), subretinal fluid (SRF), subretinal pigment epithelium fluid, and retinal, subretinal, and subretinal tissue complex thickness decreased in all treatment groups. A higher proportion of eyes treated monthly with ranibizumab had fluid resolution at 4 weeks, and the difference persisted through 52 weeks. At 52 weeks, there was little association between the presence of fluid of any type (without regard to fluid location) and the mean VA. However, at all time points, eyes with residual IRF, especially foveal IRF, had worse mean VA (9 letters) than those without IRF. Eyes with abnormally thin (<120 μm) or thick (>212 μm) retinas had worse VA than those with normal thickness (120-212 μm). At week 52, eyes with larger neovascular lesions or with foveal scar had worse VA than eyes without these features.
Conclusions: Anti-vascular endothelial growth factor (VEGF) therapy reduced lesion activity and improved VA in all treatment groups. At all time points, eyes with residual IRF had worse VA than those without. Eyes with abnormally thin or thick retinas, residual large lesions, and scar also had worse VA. Monthly ranibizumab dosing yielded more eyes with no fluid and an abnormally thin retina, although the long-term significance is unknown. These results have important treatment implications in eyes undergoing anti-VEGF therapy for neovascular AMD.
Financial disclosure(s): Proprietary or commercial disclosure may be found after the references.
Trial registration: ClinicalTrials.gov NCT00593450.
Copyright © 2013 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

