Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes
- PMID: 23642535
- PMCID: PMC3660435
- DOI: 10.1016/j.biomaterials.2013.04.026
Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes
Abstract
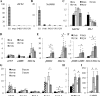
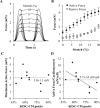
Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) provide a promising source for cell therapy and drug screening. Several high-yield protocols exist for hESC-CM production; however, methods to significantly advance hESC-CM maturation are still lacking. Building on our previous experience with mouse ESC-CMs, we investigated the effects of 3-dimensional (3D) tissue-engineered culture environment and cardiomyocyte purity on structural and functional maturation of hESC-CMs. 2D monolayer and 3D fibrin-based cardiac patch cultures were generated using dissociated cells from differentiated Hes2 embryoid bodies containing varying percentage (48-90%) of CD172a (SIRPA)-positive cardiomyocytes. hESC-CMs within the patch were aligned uniformly by locally controlling the direction of passive tension. Compared to hESC-CMs in age (2 weeks) and purity (48-65%) matched 2D monolayers, hESC-CMs in 3D patches exhibited significantly higher conduction velocities (CVs), longer sarcomeres (2.09 ± 0.02 vs. 1.77 ± 0.01 μm), and enhanced expression of genes involved in cardiac contractile function, including cTnT, αMHC, CASQ2 and SERCA2. The CVs in cardiac patches increased with cardiomyocyte purity, reaching 25.1 cm/s in patches constructed with 90% hESC-CMs. Maximum contractile force amplitudes and active stresses of cardiac patches averaged to 3.0 ± 1.1 mN and 11.8 ± 4.5 mN/mm(2), respectively. Moreover, contractile force per input cardiomyocyte averaged to 5.7 ± 1.1 nN/cell and showed a negative correlation with hESC-CM purity. Finally, patches exhibited significant positive inotropy with isoproterenol administration (1.7 ± 0.3-fold force increase, EC50 = 95.1 nm). These results demonstrate highly advanced levels of hESC-CM maturation after 2 weeks of 3D cardiac patch culture and carry important implications for future drug development and cell therapy studies.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



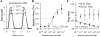
References
-
- Minami I, Yamada K, Otsuji TG, Yamamoto T, Shen Y, Otsuka S, et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012;2:1448–60. - PubMed
-
- Mummery CL, Ward D, Passier R. Differentiation of human embryonic stem cells to cardiomyocytes by coculture with endoderm in serum-free medium. Curr Protoc Stem Cell Biol. 2007 Chapter 1:Unit 1F 2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

