Protein production from the structural genomics perspective: achievements and future needs
- PMID: 23642905
- PMCID: PMC4163025
- DOI: 10.1016/j.sbi.2013.02.014
Protein production from the structural genomics perspective: achievements and future needs
Abstract
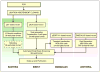
Despite a multitude of recent technical breakthroughs speeding high-resolution structural analysis of biological macromolecules, production of sufficient quantities of well-behaved, active protein continues to represent the rate-limiting step in many structure determination efforts. These challenges are only amplified when considered in the context of ongoing structural genomics efforts, which are now contending with multi-domain eukaryotic proteins, secreted proteins, and ever-larger macromolecular assemblies. Exciting new developments in eukaryotic expression platforms, including insect and mammalian-based systems, promise enhanced opportunities for structural approaches to some of the most important biological problems. Development and implementation of automated eukaryotic expression techniques promises to significantly improve production of materials for structural, functional, and biomedical research applications.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Technologies to keep an eye on: alternative hosts for protein production in structural biology.Curr Opin Struct Biol. 2013 Jun;23(3):365-73. doi: 10.1016/j.sbi.2013.02.002. Epub 2013 Mar 5. Curr Opin Struct Biol. 2013. PMID: 23481468 Review.
-
ACEMBL Tool-Kits for High-Throughput Multigene Delivery and Expression in Prokaryotic and Eukaryotic Hosts.Adv Exp Med Biol. 2016;896:27-42. doi: 10.1007/978-3-319-27216-0_3. Adv Exp Med Biol. 2016. PMID: 27165317 Review.
-
High throughput protein production for functional proteomics.Trends Biotechnol. 2003 Sep;21(9):383-8. doi: 10.1016/S0167-7799(03)00189-6. Trends Biotechnol. 2003. PMID: 12948670 Review.
-
Eukaryotic expression: developments for structural proteomics.Acta Crystallogr D Biol Crystallogr. 2006 Oct;62(Pt 10):1114-24. doi: 10.1107/S0907444906029805. Epub 2006 Sep 19. Acta Crystallogr D Biol Crystallogr. 2006. PMID: 17001089 Free PMC article.
-
Expression of recombinant glycoproteins in mammalian cells: towards an integrative approach to structural biology.Curr Opin Struct Biol. 2013 Jun;23(3):345-56. doi: 10.1016/j.sbi.2013.04.003. Epub 2013 Apr 25. Curr Opin Struct Biol. 2013. PMID: 23623336 Free PMC article. Review.
Cited by
-
Repurposing a bacterial quality control mechanism to enhance enzyme production in living cells.J Mol Biol. 2015 Mar 27;427(6 Pt B):1451-1463. doi: 10.1016/j.jmb.2015.01.003. Epub 2015 Jan 12. J Mol Biol. 2015. PMID: 25591491 Free PMC article.
-
Trends in structural coverage of the protein universe and the impact of the Protein Structure Initiative.Proc Natl Acad Sci U S A. 2014 Mar 11;111(10):3733-8. doi: 10.1073/pnas.1321614111. Epub 2014 Feb 24. Proc Natl Acad Sci U S A. 2014. PMID: 24567391 Free PMC article.
-
X-ray crystallography over the past decade for novel drug discovery - where are we heading next?Expert Opin Drug Discov. 2015;10(9):975-89. doi: 10.1517/17460441.2015.1061991. Epub 2015 Jul 15. Expert Opin Drug Discov. 2015. PMID: 26177814 Free PMC article. Review.
-
The N-terminal domain of Schmallenberg virus envelope protein Gc is highly immunogenic and can provide protection from infection.Sci Rep. 2017 Feb 13;7:42500. doi: 10.1038/srep42500. Sci Rep. 2017. PMID: 28211908 Free PMC article.
-
High-throughput cell-free screening of eukaryotic membrane protein expression in lipidic mimetics.Protein Sci. 2022 Mar;31(3):639-651. doi: 10.1002/pro.4259. Epub 2021 Dec 23. Protein Sci. 2022. PMID: 34910339 Free PMC article.
References
-
- Love J, Mancia F, Shapiro L, Punta M, Rost B, Girvin M, Wang D-N, Zhou M, Hunt JF, Szyperski T, et al. The New York Consortium on Membrane Protein Structure (NYCOMPS): a high-throughput platform for structural genomics of integral membrane proteins. J Struct Funct Genomics. 2010;11:191–199. - PMC - PubMed
-
- Sauder MJ, Rutter ME, Bain K, Rooney I, Gheyi T, Atwell S, Thompson DA, Emtage S, Burley SK. High throughput protein production and crystallization at NYSGXRC. Methods Mol Biol. 2008;426:561–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

