Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles
- PMID: 23643528
- PMCID: PMC3815987
- DOI: 10.1016/j.jconrel.2013.04.016
Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles
Abstract
The need for annual revaccination against influenza is a burden on the healthcare system, leads to low vaccination rates and makes timely vaccination difficult against pandemic strains, such as during the 2009 H1N1 influenza pandemic. In an effort toward achieving a broadly protective vaccine that provides cross-protection against multiple strains of influenza, this study developed a microneedle patch to co-immunize with A/PR8 influenza hemagglutinin DNA and A/PR8 inactivated virus vaccine. We hypothesize that this dual component vaccination strategy administered to the skin using microneedles will provide cross-protection against other strains of influenza. To test this hypothesis, we developed a novel coating formulation that did not require additional excipients to increase coating solution viscosity by using the DNA vaccine itself to increase viscosity and thereby enable thick coatings of DNA vaccine and inactivated virus vaccine on metal microneedles. Co-immunization in this way not only generated robust antibody responses against A/PR8 influenza but also generated robust heterologous antibody responses against pandemic 2009 H1N1 influenza in mice. Challenge studies showed complete cross-protection against lethal challenge with live pandemic 2009 H1N1 virus. Control experiments using A/PR8 inactivated influenza virus vaccine with placebo DNA coated onto microneedles produced lower antibody titers and provided incomplete protection against challenge. Overall, this is the first study showing DNA solution as a microneedle coating agent and demonstrating cross-protection by co-immunization with inactivated virus and DNA vaccine using coated microneedles.
Keywords: Coating; Cross-protection; DNA vaccine; Influenza virus; Microneedle.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
The resulting potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
Figures
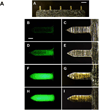

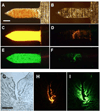



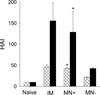

References
-
- Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. - PubMed
-
- Schwartz B, Gellin B. Vaccination strategies for an influenza pandemic. J. Infect. Dis. 2005;191:1207–1209. - PubMed
-
- Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

