Generation of integration-free and region-specific neural progenitors from primate fibroblasts
- PMID: 23643533
- PMCID: PMC3786191
- DOI: 10.1016/j.celrep.2013.04.004
Generation of integration-free and region-specific neural progenitors from primate fibroblasts
Abstract
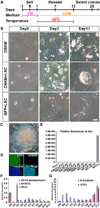
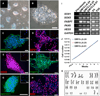
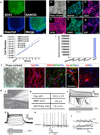
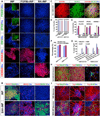
Postnatal and adult human and monkey fibroblasts were infected with Sendai virus containing the Yamanaka factors for 24 hr, then they were cultured in a chemically defined medium containing leukemia inhibitory factor (LIF), transforming growth factor (TGF)-β inhibitor SB431542, and glycogen synthase kinase (GSK)-3β inhibitor CHIR99021 at 39°C for inactivation of the virus. Induced neural progenitor (iNP) colonies appeared as early as day 13 and can be expanded for >20 passages. Under the same defined condition, no induced pluripotent stem cell (iPSC) colonies formed at either 37°C or 39°C. The iNPs predominantly express hindbrain genes and differentiate into hindbrain neurons, and when caudalized, they produced an enriched population of spinal motor neurons. Following transplantation into the forebrain, the iNP-derived cells retained the hindbrain identity. The ability to generate defined, integration-free iNPs from adult primate fibroblasts under a defined condition with predictable fate choices will facilitate disease modeling and therapeutic development.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, Takada N, Inoue M, Hasegawa M, Kawamata S, Nishikawa S. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA. 2011;108:14234–14239. - PMC - PubMed
-
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. - PubMed
-
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011;13:215–222. - PubMed
-
- Ferrari S, Griesenbach U, Iida A, Farley R, Wright AM, Zhu J, Munkonge FM, Smith SN, You J, Ban H, et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14:1371–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

