Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply?
- PMID: 23643573
- PMCID: PMC3757100
- DOI: 10.1016/j.ajog.2013.04.036
Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply?
Abstract
Objective: The continued development and use of engineered nanomaterials (ENM) has given rise to concerns over the potential for human health effects. Although the understanding of cardiovascular ENM toxicity is improving, one of the most complex and acutely demanding "special" circulations is the enhanced maternal system to support fetal development. The Barker hypothesis proposes that fetal development within a hostile gestational environment may predispose/program future sensitivity. Therefore, the objective of this study was 2-fold: (1) to determine whether maternal ENM exposure alters uterine and/or fetal microvascular function and (2) test the Barker hypothesis at the microvascular level.
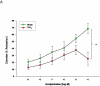
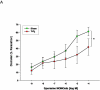
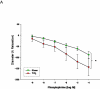
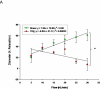
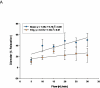
Study design: Pregnant (gestation day 10) Sprague-Dawley rats were exposed to nano-titanium dioxide aerosols (11.3 ± 0.039 mg/m(3)/hr, 5 hr/d, 8.2 ± 0.85 days) to evaluate the maternal and fetal microvascular consequences of maternal exposure. Microvascular tissue isolation (gestation day 20) and arteriolar reactivity studies (<150 μm passive diameter) of the uterine premyometrial and fetal tail arteries were conducted.
Results: ENM exposures led to significant maternal and fetal microvascular dysfunction, which was seen as robustly compromised endothelium-dependent and -independent reactivity to pharmacologic and mechanical stimuli. Isolated maternal uterine arteriolar reactivity was consistent with a metabolically impaired profile and hostile gestational environment that impacted fetal weight. The fetal microvessels that were isolated from exposed dams demonstrated significant impairments to signals of vasodilation specific to mechanistic signaling and shear stress.
Conclusion: To our knowledge, this is the first report to provide evidence that maternal ENM inhalation is capable of influencing fetal health and that the Barker hypothesis is applicable at the microvascular level.
Keywords: Barker hypothesis; engineered nanomaterials (ENM); microvascular.
Copyright © 2013 Mosby, Inc. All rights reserved.
Figures










References
-
- Borm PJ, Muller-Schulte D. Nanoparticles in drug delivery and environmental exposure: same size, same risks? Nanomedicine.(Lond) 2006;1:235–49. - PubMed
-
- National Institute of Environmental Health Sciences . Linking Early Environmental Exposures to Adult Diseases. NIEHS; 2012. 11-28-2012.
-
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Moseley AM, Cumpston JL, et al. Pulmonary Particulate Matter and Systemic Microvascular Dysfunction. Res.Rep.Health Eff.Inst. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

