Anti-hypertrophic and anti-oxidant effect of beta3-adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation
- PMID: 23643588
- PMCID: PMC4041152
- DOI: 10.1016/j.yjmcc.2013.04.025
Anti-hypertrophic and anti-oxidant effect of beta3-adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation
Abstract
Rationale: Stimulation of β3-adrenoreceptors (β3-AR) blunts contractility and improves chronic left ventricular function in hypertrophied and failing hearts in a neuronal nitric oxide synthase (nNOS) dependent manner. nNOS can be regulated by post-translational modification of stimulatory phosphorylation residue Ser1412 and inhibitory residue Ser847. However, the role of phosphorylation of these residues in cardiomyocytes and β3-AR protective signaling has yet to be explored.
Objective: We tested the hypothesis that β3-AR regulation of myocyte stress requires changes in nNOS activation mediated by differential nNOS phosphorylation.
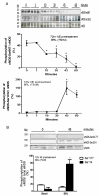
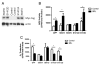
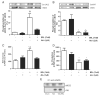
Methods and results: Endothelin (ET-1) or norepinephrine induced hypertrophy in rat neonatal ventricular cardiomyocytes (NRVMs) was accompanied by increased β3-AR gene expression. Co-administration of the β3-AR agonist BRL-37433 (BRL) reduced cell size and reactive oxygen species (ROS) generation, while augmenting NOS activity. BRL-dependent augmentation of NOS activity and ROS suppression due to NE were blocked by inhibiting nNOS (L-VNIO). BRL augmented nNOS phosphorylation at Ser1412 and dephosphorylation at Ser847. Cells expressing constitutively dephosphorylated Ser1412A or phosphorylated Ser847D nNOS mutants displayed reduced nNOS activity and a lack of BRL modulation. BRL also failed to depress ROS from NE in cells with nNOS-Ser847D. Inhibiting Akt decreased BRL-induced nNOS-Ser1412 phosphorylation and NOS activation, whereas Gi/o blockade blocked BRL-regulation of both post-translational modifications, preventing enhancement of NOS activity and ROS reduction. BRL resulted in near complete dephosphorylation of Ser847 and a moderate rise in Ser1412 phosphorylation in mouse myocardium exposed to chronic pressure-overload.
Conclusion: β3-AR regulates myocardial NOS activity and ROS via activation of nNOS involving reciprocal changes in phosphorylation at two regulatory sites. These data identify a novel and potent anti-oxidant and anti-hypertrophic pathway due to nNOS post-translational modification that is coupled to β3-AR receptor stimulation.
Keywords: Beta3-adrenergic receptors; Heart failure; Hypertrophy; Neuronal nitric oxide synthase; Reactive oxygen species.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


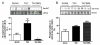

Similar articles
-
β3-Adrenoreceptor stimulation protects against myocardial infarction injury via eNOS and nNOS activation.PLoS One. 2014 Jun 9;9(6):e98713. doi: 10.1371/journal.pone.0098713. eCollection 2014. PLoS One. 2014. PMID: 24911015 Free PMC article.
-
Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase.J Am Coll Cardiol. 2012 May 29;59(22):1979-87. doi: 10.1016/j.jacc.2011.12.046. J Am Coll Cardiol. 2012. PMID: 22624839 Free PMC article.
-
Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via β3-AR-nNOS-NO activation.PLoS One. 2017 Jun 16;12(6):e0179648. doi: 10.1371/journal.pone.0179648. eCollection 2017. PLoS One. 2017. PMID: 28622359 Free PMC article.
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
-
Sub-cellular targeting of constitutive NOS in health and disease.J Mol Cell Cardiol. 2012 Feb;52(2):341-50. doi: 10.1016/j.yjmcc.2011.09.006. Epub 2011 Sep 16. J Mol Cell Cardiol. 2012. PMID: 21945464 Review.
Cited by
-
β3 Adrenergic Stimulation Restores Nitric Oxide/Redox Balance and Enhances Endothelial Function in Hyperglycemia.J Am Heart Assoc. 2016 Feb 19;5(2):e002824. doi: 10.1161/JAHA.115.002824. J Am Heart Assoc. 2016. PMID: 26896479 Free PMC article.
-
β3-adrenoceptor impacts apoptosis in cultured cardiomyocytes via activation of PI3K/Akt and p38MAPK.J Huazhong Univ Sci Technolog Med Sci. 2016 Feb;36(1):1-7. doi: 10.1007/s11596-016-1533-7. Epub 2016 Feb 3. J Huazhong Univ Sci Technolog Med Sci. 2016. PMID: 26838732
-
Role of the β3-adrenergic receptor subtype in catecholamine-induced myocardial remodeling.Mol Cell Biochem. 2018 Sep;446(1-2):149-160. doi: 10.1007/s11010-018-3282-3. Epub 2018 Jan 23. Mol Cell Biochem. 2018. PMID: 29363058
-
Comprehensive Mechanism, Novel Markers and Multidisciplinary Treatment of Severe Acute Pancreatitis-Associated Cardiac Injury - A Narrative Review.J Inflamm Res. 2021 Jul 12;14:3145-3169. doi: 10.2147/JIR.S310990. eCollection 2021. J Inflamm Res. 2021. PMID: 34285540 Free PMC article. Review.
-
Neuronal nitric oxide synthase in hypertension - an update.Clin Hypertens. 2016 Nov 3;22:20. doi: 10.1186/s40885-016-0055-8. eCollection 2016. Clin Hypertens. 2016. PMID: 27822383 Free PMC article. Review.
References
-
- Arch JR, Ainsworth AT. Thermogenic and antiobesity activity of a novel beta-adrenoceptor agonist (BRL 26830A) in mice and rats. Am J Clin Nutr. 1983;38:549–58. - PubMed
-
- Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–55. - PubMed
-
- Germack R, Dickenson JM. Induction of beta3-adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J Pharmacol Exp Ther. 2006;316:392–406. - PubMed
-
- Angelone T, Filice E, Quintieri AM, Imbrogno S, Recchia A, Pulera E, et al. Beta3-adrenoceptors modulate left ventricular relaxation in the rat heart via the NO-cGMP-PKG pathway. Acta Physiol (Oxf) 2008;193:229–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

