A truncated splice-variant of the FcεRIβ receptor subunit is critical for microtubule formation and degranulation in mast cells
- PMID: 23643722
- PMCID: PMC3694348
- DOI: 10.1016/j.immuni.2013.04.007
A truncated splice-variant of the FcεRIβ receptor subunit is critical for microtubule formation and degranulation in mast cells
Abstract
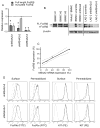
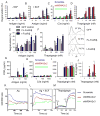
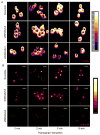
Human linkage analyses have implicated the MS4A2-containing gene locus (encoding FcεRIβ) as a candidate for allergy susceptibility. We have identified a truncation of FcεRIβ (t-FcεRIβ) in humans that contains a putative calmodulin-binding domain and thus, we sought to identify the role of this variant in mast cell function. We determined that t-FcεRIβ is critical for microtubule formation and degranulation and that it may perform this function by trafficking adaptor molecules and kinases to the pericentrosomal and Golgi region in response to Ca2+ signals. Mutagenesis studies suggest that calmodulin binding to t-FcεRIβ in the presence of Ca2+ could be critical for t-FcεRIβ function. In addition, gene targeting of t-FcεRIβ attenuated microtubule formation, degranulation, and IL-8 production downstream of Ca2+ signals. Therefore, t-FcεRIβ mediates Ca2+ -dependent microtubule formation, which promotes degranulation and cytokine release. Because t-FcεRIβ has this critical function, it represents a therapeutic target for the downregulation of allergic inflammation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. - PubMed
-
- Cookson WO, Sharp PA, Faux JA, Hopkin JM. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet. 1989;1:1292–1295. - PubMed
-
- Donnadieu E, Cookson WO, Jouvin MH, Kinet JP. Allergy-associated polymorphisms of the FcεRIβ subunit do not impact its two amplification functions. J Immunol. 2000a;165:3917–3922. - PubMed
-
- Donnadieu E, Jouvin MH, Kinet JP. A second amplifier function for the allergy-associated FcεRI-β subunit. Immunity. 2000b;12:515–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

