Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine
- PMID: 23643743
- PMCID: PMC3696421
- DOI: 10.1016/j.neuropharm.2013.04.040
Cyclooxygenase activity contributes to the monoaminergic damage caused by serial exposure to stress and methamphetamine
Abstract
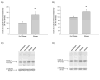
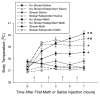
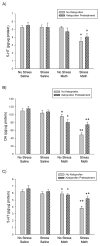
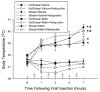
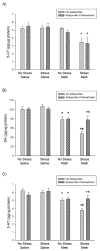
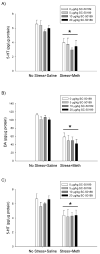
Methamphetamine (Meth) is a widely abused psychostimulant that causes long-term dopamine (DA) and serotonin (5-HT) depletions. Stress and Meth abuse are comorbid events in society and stress exacerbates Meth-induced monoaminergic terminal damage. Stress is also known to produce neuroinflammation. This study examined the role of the neuroinflammatory mediator, cyclooxygenase (COX), in the depletions of monoamines caused by serial exposure to chronic unpredictable stress (CUS) and Meth. CUS produced an increase in COX-2 protein expression and enhanced Meth-induced monoaminergic depletions in the striatum and hippocampus. The enhanced DA and 5-HT depletions in the striatum, but not the hippocampus, were prevented by pretreatment with COX inhibitor, ketoprofen, during stress or during Meth; however, ketoprofen did not attenuate the monoaminergic damage caused by Meth alone. The COX-dependent enhancement by stress of Meth-induced monoaminergic depletions was independent of hyperthermia, as ketoprofen did not attenuate Meth-induced hyperthermia. In addition, the EP1 receptor antagonist, SC-51089, did not attenuate DA or 5-HT depletions caused by stress and Meth. These findings illustrate that COX activity, but not activation of the EP1 receptor, is responsible for the potentiation of Meth-induced damage to striatal monoamine terminals by stress and suggests the use of anti-inflammatory drugs for mitigating the neurotoxic effects associated with the combination of stress and Meth.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier.J Neuroimmune Pharmacol. 2012 Dec;7(4):951-68. doi: 10.1007/s11481-012-9391-y. Epub 2012 Jul 26. J Neuroimmune Pharmacol. 2012. PMID: 22833424 Free PMC article.
-
Augmentation of methamphetamine-induced toxicity in the rat striatum by unpredictable stress: contribution of enhanced hyperthermia.Eur J Neurosci. 2007 Aug;26(3):739-48. doi: 10.1111/j.1460-9568.2007.05688.x. Eur J Neurosci. 2007. PMID: 17686046
-
Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum.J Pharmacol Exp Ther. 2005 Jan;312(1):160-9. doi: 10.1124/jpet.104.072264. Epub 2004 Aug 26. J Pharmacol Exp Ther. 2005. PMID: 15331654
-
Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways.Psychopharmacology (Berl). 2016 May;233(10):1945-62. doi: 10.1007/s00213-016-4235-8. Epub 2016 Feb 12. Psychopharmacology (Berl). 2016. PMID: 26873080 Free PMC article. Review.
-
Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption.J Pharmacol Sci. 2003 Jul;92(3):178-95. doi: 10.1254/jphs.92.178. J Pharmacol Sci. 2003. PMID: 12890883 Review.
Cited by
-
Effects of sequential ethanol exposure and repeated high-dose methamphetamine on striatal and hippocampal dopamine, serotonin and glutamate tissue content in Wistar rats.Neurosci Lett. 2018 Feb 5;665:61-66. doi: 10.1016/j.neulet.2017.11.043. Epub 2017 Nov 22. Neurosci Lett. 2018. PMID: 29174641 Free PMC article.
-
Chronic voluntary oral methamphetamine induces deficits in spatial learning and hippocampal protein kinase Mzeta with enhanced astrogliosis and cyclooxygenase-2 levels.Heliyon. 2018 Feb 2;4(2):e00509. doi: 10.1016/j.heliyon.2018.e00509. eCollection 2018 Feb. Heliyon. 2018. PMID: 29560440 Free PMC article.
-
Repeated Methamphetamine Administration Results in Axon Loss Prior to Somatic Loss of Substantia Nigra Pars Compacta and Locus Coeruleus Neurons in Male but Not Female Mice.Int J Mol Sci. 2023 Aug 22;24(17):13039. doi: 10.3390/ijms241713039. Int J Mol Sci. 2023. PMID: 37685846 Free PMC article.
-
Cerebrovascular Injury After Serial Exposure to Chronic Stress and Abstinence from Methamphetamine Self-Administration.Sci Rep. 2018 Jul 12;8(1):10558. doi: 10.1038/s41598-018-28970-1. Sci Rep. 2018. PMID: 30002494 Free PMC article.
-
Role of microglia in methamphetamine-induced neurotoxicity.Int J Physiol Pathophysiol Pharmacol. 2017 Jun 15;9(3):84-100. eCollection 2017. Int J Physiol Pathophysiol Pharmacol. 2017. PMID: 28694920 Free PMC article. Review.
References
-
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett. 2003;352:13–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

