Cytotoxicity, genotoxicity, and mutagenicity of 1-chloro-2-hydroxy-3-butene and 1-chloro-3-buten-2-one, two alternative metabolites of 1,3-butadiene
- PMID: 23643860
- PMCID: PMC3714330
- DOI: 10.1016/j.taap.2013.04.019
Cytotoxicity, genotoxicity, and mutagenicity of 1-chloro-2-hydroxy-3-butene and 1-chloro-3-buten-2-one, two alternative metabolites of 1,3-butadiene
Abstract
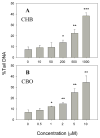
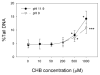
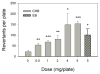
The cytotoxicity, genotoxicity, and mutagenicity of 1-chloro-2-hydroxy-3-butene (CHB), a known in vitro metabolite of the human carcinogen 1,3-butadiene, have not previously been investigated. Because CHB can be bioactivated by alcohol dehydrogenases to yield 1-chloro-3-buten-2-one (CBO), a bifunctional alkylating agent that caused globin-chain cross-links in erythrocytes, in the present study we investigated the cytotoxic and genotoxic potential of CHB and CBO in human normal hepatocyte L02 cells using the MTT assay, the relative cloning efficiency assay and the comet assay. We also investigated the mutagenic potential of these compounds with the Ames test using Salmonella strains TA1535 and TA1537. The results provide clear evidence for CHB and CBO being both cytotoxic and genotoxic with CBO being approximately 100-fold more potent than CHB. Interestingly, CHB generated both single-strand breaks and alkali-labile sites on DNA, whereas CBO produced only alkali-labile sites. CHB did not directly result in DNA breaks, whereas CBO was capable of directly generating breaks on DNA. Interestingly, both compounds did not induce DNA cross-links as examined by the comet assay. The Ames test results showed that CHB induced point mutation but not frameshift mutation, whereas the toxic effects of CBO made it difficult to reliably assess the mutagenic potential of CBO in the two strains. Collectively, the results suggest that CHB and CBO may play a role in the mutagenicity and carcinogenicity of 1,3-butadiene.
Keywords: %Tail DNA; 1,2,3,4-diepoxybutane; 1,3-Butadiene; 1,3-butadiene; 1-Chloro-2-hydroxy-3-butene; 1-Chloro-3-buten-2-one; 1-chloro-2-hydroxy-3-butene; 1-chloro-3-buten-2-one; 3,4-epoxy-1,2-butanediol; 3,4-epoxy-1-butene; 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; ALS; BD; CBO; CHB; Comet assay; DEB; EB; EBD; FBS; GSH; Genotoxicity; MMS; MTT; Mutagenicity; RCE; SD; SSB; alkali-labile sites; fetal bovine serum; glutathione; methyl methanesulfonate; percentage of DNA in the tail; relative cloning efficiency; single-strand breaks; standard deviation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
DNA damage induced by three major metabolites of 1,3-butadiene in human hepatocyte L02 cells.Mutat Res. 2012 Sep 18;747(2):240-5. doi: 10.1016/j.mrgentox.2012.06.001. Epub 2012 Jun 12. Mutat Res. 2012. PMID: 22698567
-
Alcohol dehydrogenase- and rat liver cytosol-dependent bioactivation of 1-chloro-2-hydroxy-3-butene to 1-chloro-3-buten-2-one, a bifunctional alkylating agent.Chem Res Toxicol. 2012 Nov 19;25(11):2600-7. doi: 10.1021/tx300369b. Epub 2012 Nov 7. Chem Res Toxicol. 2012. PMID: 23110628 Free PMC article.
-
Bioactivation of 1-chloro-2-hydroxy-3-butene, an in vitro metabolite of 1,3-butadiene, by rat liver microsomes.Chem Biol Interact. 2018 Feb 25;282:36-44. doi: 10.1016/j.cbi.2018.01.006. Epub 2018 Jan 10. Chem Biol Interact. 2018. PMID: 29329665
-
A review of the genetic and related effects of 1,3-butadiene in rodents and humans.Mutat Res. 2000 Oct;463(3):181-213. doi: 10.1016/s1383-5742(00)00056-9. Mutat Res. 2000. PMID: 11018742 Review.
-
Insights into the toxicokinetics and toxicodynamics of 1,3-butadiene.Chem Biol Interact. 2001 Jun 1;135-136:599-614. doi: 10.1016/s0009-2797(01)00199-5. Chem Biol Interact. 2001. PMID: 11397415 Review.
Cited by
-
Formation of fused-ring 2'-deoxycytidine adducts from 1-chloro-3-buten-2-one, an in vitro 1,3-butadiene metabolite, under in vitro physiological conditions.Chem Res Toxicol. 2013 Oct 21;26(10):1545-53. doi: 10.1021/tx4002435. Epub 2013 Sep 25. Chem Res Toxicol. 2013. PMID: 24020501 Free PMC article.
-
Concentration- and time-dependent genotoxicity profiles of isoprene monoepoxides and diepoxide, and the cross-linking potential of isoprene diepoxide in cells.Toxicol Rep. 2014 Mar 28;1:36-45. doi: 10.1016/j.toxrep.2014.03.002. eCollection 2014. Toxicol Rep. 2014. PMID: 28962224 Free PMC article.
-
1,3-Butadiene: a ubiquitous environmental mutagen and its associations with diseases.Genes Environ. 2022 Jan 10;44(1):3. doi: 10.1186/s41021-021-00233-y. Genes Environ. 2022. PMID: 35012685 Free PMC article. Review.
References
-
- Adler ID, Kliesch U, Nylund L, Poltonen K. In vitro and in vivo mutagenicity of the butadiene metabolites butadiene diolepoxide, butadiene monoepoxide and diepoxybutane. Mutagenesis. 1997;12:339–345. - PubMed
-
- Alary J, Fernandez Y, Debrauwer L, Perdu E, Gueraud F. Identification of intermediate pathways of 4-hydroxynonenal metabolism in the rat. Chem Res Toxicol. 2003;16:320–327. - PubMed
-
- Albertini RJ, Carson ML, Kirman CR, Gargas ML. 1,3-Butadiene: II. Genotoxicity profile Crit Rev Toxicol. 2010;40(S1):12–73. - PubMed
-
- Boogaard PJ, Bond JA. The role of hydrolysis in the detoxification of 1,2:3,4-diepoxybutane by human, rat, and mouse liver and lung in vitro. Toxicol Appl Pharmacol. 1996;141:617–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

