Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells
- PMID: 23643939
- PMCID: PMC3712508
- DOI: 10.1016/j.ydbio.2013.04.023
Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells
Abstract
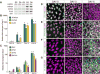
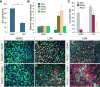
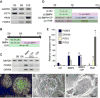
Sensory and endoneurocrine tissues as diverse as the lens, the olfactory epithelium, the inner ear, the cranial sensory ganglia, and the anterior pituitary arise from a common pool of progenitors in the preplacodal ectoderm (PPE). Around late gastrulation, the PPE forms at the border surrounding the anterior neural plate, and expresses a unique set of evolutionarily conserved transcription regulators including Six1, Eya 1 and Eya2. Here, we describe the first report to generate and characterize the SIX1(+) PPE cells from human embryonic stem (ES) cells by adherent differentiation. Before forming PPE cells, differentiating cultures first expressed the non-neural ectoderm specific transcriptional factors TFAP2A, GATA2, GATA3, DLX3, and DLX5, which are crucial in establishing the PPE competence. We demonstrated that bone morphogenetic protein (BMP) activity plays a transient but essential role in inducing expression of these PPE competence factors and eventually the PPE cells. Interestingly, we found that attenuating BMP signaling after establishing the competence state induces anterior placode precursors. By manipulating BMP and hedgehog signaling pathways, we further differentiate these precursors into restricted lineages including the lens placode and the oral ectoderm (pituitary precursor) cells. Finally, we also show that sensory neurons can be generated from human PPE cells, demonstrating the multipotency of the human ES-derived PPE cells.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Transcriptional regulation of cranial sensory placode development.Curr Top Dev Biol. 2015;111:301-50. doi: 10.1016/bs.ctdb.2014.11.009. Epub 2015 Jan 22. Curr Top Dev Biol. 2015. PMID: 25662264 Free PMC article. Review.
-
Specific induction of cranial placode cells from Xenopus ectoderm by modulating the levels of BMP, Wnt, and FGF signaling.Genesis. 2015 Oct;53(10):652-9. doi: 10.1002/dvg.22881. Epub 2015 Aug 24. Genesis. 2015. PMID: 26249012
-
A gene network that coordinates preplacodal competence and neural crest specification in zebrafish.Dev Biol. 2013 Jan 1;373(1):107-17. doi: 10.1016/j.ydbio.2012.10.012. Epub 2012 Oct 16. Dev Biol. 2013. PMID: 23078916 Free PMC article.
-
Early embryonic specification of vertebrate cranial placodes.Wiley Interdiscip Rev Dev Biol. 2014 Sep-Oct;3(5):349-63. doi: 10.1002/wdev.142. Epub 2014 Jul 2. Wiley Interdiscip Rev Dev Biol. 2014. PMID: 25124756 Review.
-
Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4.Dev Biol. 2009 Feb 1;326(1):75-85. doi: 10.1016/j.ydbio.2008.10.039. Epub 2008 Nov 7. Dev Biol. 2009. PMID: 19027001 Free PMC article.
Cited by
-
Transcriptional regulation of cranial sensory placode development.Curr Top Dev Biol. 2015;111:301-50. doi: 10.1016/bs.ctdb.2014.11.009. Epub 2015 Jan 22. Curr Top Dev Biol. 2015. PMID: 25662264 Free PMC article. Review.
-
Single-cell analysis delineates a trajectory toward the human early otic lineage.Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):8508-13. doi: 10.1073/pnas.1605537113. Epub 2016 Jul 11. Proc Natl Acad Sci U S A. 2016. PMID: 27402757 Free PMC article.
-
Signaling mechanisms controlling cranial placode neurogenesis and delamination.Dev Biol. 2014 May 1;389(1):39-49. doi: 10.1016/j.ydbio.2013.11.025. Epub 2013 Dec 3. Dev Biol. 2014. PMID: 24315854 Free PMC article. Review.
-
Inner ear hair cell-like cells from human embryonic stem cells.Stem Cells Dev. 2014 Jun 1;23(11):1275-84. doi: 10.1089/scd.2014.0033. Epub 2014 Mar 10. Stem Cells Dev. 2014. PMID: 24512547 Free PMC article.
-
Enriched Differentiation of Human Otic Sensory Progenitor Cells Derived From Induced Pluripotent Stem Cells.Front Mol Neurosci. 2018 Dec 20;11:452. doi: 10.3389/fnmol.2018.00452. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30618604 Free PMC article.
References
-
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Developmental biology. 2005;288:40–59. - PubMed
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental biology. 2000;227:271–278. - PubMed
-
- Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Developmental biology. 2003;257:1–13. - PubMed
-
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. - PubMed
-
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Molecular reproduction and development. 2008;75:818–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

