OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells
- PMID: 23643940
- PMCID: PMC3892773
- DOI: 10.1016/j.canlet.2013.04.027
OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells
Abstract
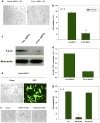
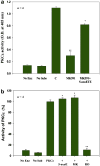
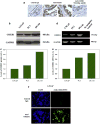
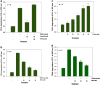
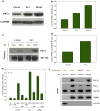
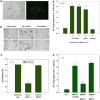
Inhibition of 5-Lox induces apoptosis in prostate cancer cells by inactivating PKCε which is prevented by 5-oxoETE, and activators of PKCε prevent 5-Lox inhibition-induced apoptosis, suggesting that 5-Lox metabolites exert survival signaling via PKCε. However, mechanisms by which 5-Lox metabolites activate PKCε are not understood yet. We found that prostate cancer cells express high levels of OXER1, a G protein-coupled 5-oxoETE receptor, which delivers signal by generating diacyl-glycerol through phospholipase C-beta. Interestingly, we found that U73122, an inhibitor of PLC-beta, interrupts the apoptosis-preventing effect of 5-oxoETE, and exogenous diacyl-glycerol effectively prevents 5-Lox inhibition-induced apoptosis, suggesting that 5-oxoETE signals via OXER1 to promote prostate cancer cell survival.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures


References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Fleshner N, Bagnell PS, Klotz L, Venkateswaran V. Dietary fat and prostate cancer. J Urol. 2004;171:19–24. - PubMed
-
- Kolonel LN, Nomura AN, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–428. - PubMed
-
- Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456–1462. - PubMed
-
- West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

